| Issue |
Wuhan Univ. J. Nat. Sci.
Volume 30, Number 2, April 2025
|
|
|---|---|---|
| Page(s) | 205 - 212 | |
| DOI | https://doi.org/10.1051/wujns/2025302205 | |
| Published online | 16 May 2025 | |
Chemistry
CLC number: O657.3
High-Efficiency Detection for Silver Ions Based on Fluorescence Enhancement of Peptide-Gold Nanoparticles
基于多肽-金纳米粒子体系荧光增强的银离子高效检测
Core Facility of Wuhan University, Wuhan University, Wuhan 430072, Hubei, China
† Corresponding author. E-mail: zhouxd@whu.edu.cn; jmhu@whu.edu.cn
Received:
12
September
2024
Silver ion (Ag+) is a highly toxic metal ion, and its monitoring in water or food resources has become extraordinarily necessary within the scope of human health. In the light of the fact of Ag+-induced folding structure of specific peptides, an unlabeled and high-selectivity Ag+ assay is presented by means of intrinsic fluorescence of peptides. Under the quenching effect of gold nanoparticles (AuNPs), characteristic fluorescence of peptides could be considerably reduced by rapid modification. Along with the Ag+ adding, the fluorescence signals of peptide-AuNPs are largely enhanced by the behavior between peptides and Ag+. This is basically involving the formation of 4-coordinated complexes, generating the changes of peptides in structure and fluorescence properties. Under this circumstance, the adverse influence of plenty of interfering ions is suppressed, including the toxic Hg2+, Pb2+. The results highlight that Ag+ ions could be selectively recognized as low as 2.4 nmol/L with a linear range of 5 to 800 nmol/L. In comparison with other programs, the given approach declares simplicity, sensitivity, and superior selectivity. Furthermore, the biosensor excels in the practical application in water samples (e.g., lake, tap and drinking water) owing to its non-interference and on-site rapid determination.
摘要
银离子是一种剧毒的金属离子,其检测对于人类的健康安全至关重要。根据银离子诱导特定多肽折叠这一理论基础,我们利用多肽自身的固有荧光,提出了一种无标记、高选择性的银离子测定方法。借助于金纳米粒子的猝灭作用,多肽的固有荧光能得到显著降低。而随着银离子的加入,多肽逐渐与银离子相互作用,发生构型上的变化,此时多肽-金纳米粒子复合体系的荧光信号得以恢复。这些信号变化主要与多肽-银离子形成的四配位化合物有关,从而使多肽的结构与荧光信号发生了明显变化。基于上述原理,该传感体系表现出良好的选择性,并不会受其他离子(包括汞离子和铅离子)的干扰。实验结果表明,该体系对银离子具备高识别性与高专一性,在5 ~ 800 nmol/L的线性范围内可特异性识别银离子,其检出限可低至2.4 nmol/L。与其他传感体系相比,该方法具有简单快速、灵敏高效、选择性好、具备现场快速测定等优势,在水样(如湖泊、自来水和饮用水)的检测中有着广泛的应用前景。
Key words: fluorescence assay / peptide-AuNPs / Ag+ detection / quenching / fluorescence recovery
关键字 : 荧光检测 / 多肽-金纳米粒子复合体系 / 银离子检测 / 猝灭作用 / 荧光恢复
Cite this article: LI Xinyi, ZHOU Xiaodong, HU Jiming. High-Efficiency Detection for Silver Ions Based on Fluorescence Enhancement of Peptide-Gold Nanoparticles[J]. Wuhan Univ J of Nat Sci, 2025, 30(2): 205-212.
Biography: LI Xinyi, female, Ph.D. candidate, research direction: biosensors. E-mail: xinyilee@whu.edu.cn
Foundation item: Supported by the National Natural Science Foundation of China (21775114, 21874102)
© Wuhan University 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
0 Introduction
Silver ion (Ag+) is an essential form of silver, which is always unrestrictedly released into the environment from industrial wastes. It is one of the most hazardous pollutants, which adversely affects the environment and has serious biological effects on human health. Exposed to Ag+ may lead to argyria and severe symptoms such as headache, skin irritation, stomach distress, organ edema, and even death. The mechanism is that Ag binds with many metabolites, inactivates sulfhydryl enzymes, and then induces various disorders[1-3]. Accordingly, accurate detection of Ag+ is of great significance and has become a hot topic in scientific research.
Traditional techniques for Ag+ assays require a sophisticated apparatus such as atomic absorption/emission spectroscopy and inductively coupled plasma mass spectroscopy (ICPMS)[4-7]. However, the shortcomings of high cost, low selectivity, and complicated operations hamper their applications. With the development of nanotechnology and coordination reactions, sensitive Ag+ sensors with regard to biomolecular recognizer(e.g., aptamers, oligonucleotides, peptides, proteins) have been fabricated in response to the defects of traditional techniques[8-11]. Much effort has been devoted toward establishing DNA-mediated Ag+ sensors on the foundation of interactions between Ag+ and basic groups. Yang et al[12] adopted a novel approach using G-quadruplex-specific fluorescence enhancement of thioflavin T for the Ag+ determination. Lin et al[13] presented a DNA sensor for simultaneous detection of Pb2+, Ag+, and Hg2+ by electrochemical impedance spectroscopy (EIS) with [Fe(CN)6]4-/3- as redox probe. Although these assays are effective, most of them suffer extra labels, complex purification and modification steps[14-17]. As a consequence, a suitable recognition unit to develop a simple, advanced and unlabeled Ag+ detection is highly desired.
Recently, considerable attention has been paid to peptides, who possess great flexibility and versatility in structural and chemical properties[18]. Their utilization has accelerated the development of nanomaterials and biopolymers. Furthermore, the behaviors of peptides and metal ions can offer the possibility to construct a biosensor because of their high affinity and selectivity. Previously, our group has projected the colorimetric assay for Ag+ regarding peptides-modified gold nanoparticles (peptide-AuNPs), which mainly relies on the 4-coordination of peptides and Ag+ [19]. The peptide has two free —COOH groups and two free —NH2 groups in the side chain owing to the aspartic acids and arginines, which could form the 4-coordinated complex with Ag+ to further induce the aggregation of AuNPs. In addition, MALDI-TOF MS experiments proved the Ag+-induced folding structure of peptides with no distractions of other metal ions. In spite of the simplicity and sensitivity, this testing anlysis is subjected to intense interference from Hg2+. To combat this situation, we devote to propose a new peptides-mediated Ag+ detection platform, achieving the optimization of the selectivity and sensitivity. In recent years, fluorescent sensing technology has attracted more and more attention due to its advantages of simple operation and rapid detection[20-23]. It is acknowledged that, with the existing of three kinds of unique amino acids (tryptophan, tyrosine and phenylalanine), these peptides would produce the intrinsic fluorescence[23-26]. The lowest fluorescence quantum yields of phenylalanine are preferred to form a "turn-off" fluorescence detection mode. Another evident fact is that some metal ions have potential to boost the fluorescence of molecules by complexing action, especially Ag+, Zn2+, Cu2+[27-31].
Taking the findings into account, we herein report a rapid, unlabeled and high-selectivity determination of Ag+ based on enhanced intrinsic fluorescence of specific peptides (Fig. 1). In the case, a peptide (RFPRGGDD) containing phenylalanine and the 4-coordinated sites of Ag+ is employed. And then, AuNPs are engineered to eliminate the peptides fluorescence as the quenching agent, owing to their huge reactive surface and quenching ability. We simply utilize the unique behavior to promote the complexation of peptides and Ag+. It generates strong fluorescence signals, from which Ag+ can be easily recognized and quantified. In light of the improved measurement mechanism, the disturbance of Hg2+ in detecting system are thoroughly suppressed. We further demonstrate the analytical potential of this method for monitoring Ag+ in water samples. Compared with several approaches, our proposal paves a distinctive avenue to monitor Ag+ in consideration of its simplicity, rapidity, superior selectivity.
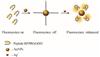 |
Fig. 1 Schematic illustrations of Ag+-enhanced fluorescence of peptide-AuNPs |
1 Experimental
1.1 Materials
Tri-sodium citrate (C6H5Na3O7), HAuCl4·3H2O, LiCl, KCl, MgCl2, CaCl2, Pb(NO3)2, NaCl, NH4Cl, FeCl2, Fe(NO3)3, Hg(NO3)2, H3BO3, CrCl3, MnCl2, FeCl3, CoCl2, NiCl2, CuCl2, ZnCl2, AgNO3, KNO3, K2SO4, NaAc, NaNO2, NaF, NaI, NaBr, NaOH, Na2HPO4, NaH2PO4, K2CO3 and NaHCO3 were purchased from Sinopharm Chemical Reagent (China). Peptide Arg-Phe-Pro-Arg-Gly-Gly-Asp-Asp (RFPRGGDD) were 95% pure and obtained from Sangon Biotechnology Inc. (Shanghai, China). In our assay, phosphate buffer (PB) was prepared as the buffer. All solutions and the buffers in the experiments were obtained using ultrapure water.
1.2 Instrumentations
The absorption spectra of AuNPs were recorded by a Shimadzu UV-2550. Fluorescence measurements were performed on a F-4600 spectrometer (Hitachi Co. Ltd., Japan) with a xenon lamp excitation source. An Eppendorf centrifuge 5415R was used for centrifugation of the AuNPs. The size distribution and surface potential were measured with a Zetasizer Nano ZS (Malvern Instruments).
1.3 Synthesis of AuNPs
As previously published methods, AuNPs of different sizes were obtained by reduction of the HAuCl4 salt[19]. Obviously, 50 mL of 0.01% (W/W) HAuCl4 was heated by boiling under vigorous magnetic stirring, then adding 11.4 mg/mL sodium citrate solution. Accordingly, the addition of 1.5, 1.0 and 0.75 mL sodium citrate solution contributed to the synthesis of 15, 20 and 30 nm AuNPs, respectively. The mixture was kept boiling and magnetic stirring for 20 min after turning red, and then cooled to room temperature. Finally, AuNPs with different sizes were prepared and stored at 4 ℃.
1.4 Fluorescence Detection of Ag+
Briefly, peptide RFPRGGDD (0.1 mmol/L) was dissolved in PB (10 mmol/L, pH 7.4). Then, 100 µL of AuNPs was added to peptide (200 µL). The reaction mixture (peptide-AuNPs) was incubated at room temperature for several minutes.
AgNO3 stock solution was prepared for the Ag+ assay. Diverse concentrations of Ag+ were obtained by serially diluting the stock solution to test the sensitivity limits. Then, 150 µL of mixture solution was added 50 µL of Ag+ with different concentrations. Therefore, the final volume of the reaction mixture was regulated to 200 µL. The changes of fluorescence intensity at 560 nm correspond to the quantities of Ag+, which could be detected by florescence spectrophotometer with EX 279 nm and EM 560 nm. All experiments were repeated three times, respectively.
1.5 Selectivity and Recovery Test for Ag+
In the experiments for selectivity and practical assay, all samples were tested in the above-mentioned conditions. We explored the selectivity over other potential common ions (Cu2+, Ni2+, Pb2+, Co2+, Zn2+, Ca2+, Mn2+, Mg2+, K+, Fe2+, Fe3+, Cr3+, Ag+, Na+, NH4+, Ba2+, Hg2+, Li+, SO42-, Ac-, NO2-, NO3-, F-, CO32-, HCO3-, H2PO4-, HPO42-, H4BO4-, OH-, Cl-, I-, Br-). Besides that, the influence of these anions on Ag+ binding to peptide-AuNPs was investigated.
The recovery experiments were accomplished using Ag+-spiked lake water, tap water and drinking water. Compared with the standard curve of Ag+, we confirmed the Ag+ concentration in the samples by using the fluorescence response of Ag+-spiked water samples. Consequently, the relevant recovery values were calculated.
2 Results and Discussion
2.1 Evidences of AuNPs-Quenched Fluorescence of Peptide
Considering the unique structure, peptides RFPRGGDD not only possess the intrinsic fluorescence, but also have great capacity to coordinate with Ag+, thus permitting access to the changes of fluorescence signals[19]. When it comes to testing principle, AuNPs must be introduced to quench the peptides fluorescence for the sake of low background interference and high sensitivity. Theoretically, the peptide RFPRGGDD can be immobilized to the surface of AuNPs through Au-N bonds with the assistance of its available —NH2. And a uniformly charged layer of nanoparticles would be constituted to facilitate the stability of peptide-AuNPs system, resulting from the low isoelectric point of peptide. This trend provides the opportunity for the quenching effect of AuNPs on peptides. To ensure the behavior, the fluorescence spectrum of peptides and UV-vis spectrum of AuNPs were respectively investigated. In Fig. 2, it could be observed that the absorption spectrum of AuNPs and the emission spectrum of peptides were largely overlapped, which met the requirement of fluorescence resonance energy transfer (FRET) well. Accordingly, the quenching action between peptides and AuNPs could be realized.
 |
Fig. 2 UV-vis spectra of AuNPs and fluorescence emission spectra of peptides RFPRGGDD |
2.2 Parameters Optimization
Several parameters including the AuNPs size and consumption, the reaction time, the pH and the PB concentration, were estimated. The signal change (F/F0) was an essential indictor to evaluate the quenching procedure, and F0 and F respectively represented the fluorescence of peptides in absence and presence of AuNPs. As shown in Fig. 3(a), the best performance came from 20 nm AuNPs. When the reaction was run in 50 s, saturation was reached (Fig. 3(b)).
 |
Fig. 3 Effects of AuNPs size (a), reaction time (b), pH(c), PB concentration (d) on quenching fluorescence of peptides |
The impact of pH on AuNPs-quenched fluoresence of peptide was explored as well. In Fig. 3(c), with the increase of pH, the ratio of emission fluorescence (F/F0) was increased at the early stage and then decreased. And the maximum appeared when the pH value was 7.0. This is mainly because pH dominates the stability of peptide-AuNPs complexes, further affecting their fluorescence transfer process. Similarly, PB concentration plays a key role in fluorescence signals due to the influence of the ion strength on system stability. The variation of F/F0 at different PB concentrations was shown in Fig. 3(d). It was evident that 10 mmol/L of PB concentration was optimal in the given project.
2.3 Fluorescence Assay for Rapid Ag+ Sensing
To evaluate the Ag+ sensing performance of this peptide-AuNPs system, different concentrations of Ag+ solutions ranging from 2 to 1 000 nmol/L were prepared. When increased Ag+ concentrations were added into the peptide-AuNPs complexes, fluorescence intensity of the solution was gradually enhanced (Fig. 4(a)). The curves indicated that the fluorescence recovery largely depended on Ag+ concentrations. We used the ratio of fluorescence (F/F1) to express the degree of enhancement, and F1 and F respectively represented the fluorescence of peptide-AuNPs complexes in absence and presence of Ag+. Figure 4(b) gave an explanation about the relationship between Ag+ concentration and the ratio value of F/F1. The F/F1 values apparently raised with the Ag+ concentrations from 2 to 1 000 nmol/L. Once Ag+ concentrations increased to 800 nmol/L, the signal variations were slight. Definitely, the inset of Fig. 4(b) suggested that the response of the assay was extremely linear, with a linear regression correlation coefficient of 0.999 at the Ag+ concentrations range of 5 to 800 nmol/L. The detection limit of Ag+ with our system is proposed to be 2.4 nmol/L, which is significantly below the allowed Ag+ concentration limit (460 nmol/L) defined by the United States Environmental Protection Agency (USEPA) in drinkable water[13]. Aside from that, the Ag+ testing time was surveyed. Figure 4(c) presented the fluorescence kinetics analysis of peptide-AuNPs complexes at different Ag+ concentrations. The results revealed that the whole detection could be fully achieved in 10 min.
 |
Fig. 4 Ag+ sensing performance of the peptide-AuNPs system (a) Fluorescence spectra of peptide-AuNPs with different Ag+ concentra-tions; (b) Responses (F/F1) of peptide-AuNPs with different Ag+ con-centrations, and inset shows the response linearity of the assay at the Ag+ concentrations range of 5 to 800 nmol/L; (c) Reaction time of peptide-AuNPs with Ag+. |
2.4 Selectivity for Determination of Ag+
In order to identify the selectivity of the assay, 18 cations (Al3+, Ni2+, Pb2+, Co2+, Zn2+, Ca2+, Mn2+, Mg2+, K+, Fe2+, Fe3+, Cr3+, Ag+, Na+, NH4+, Ba2+, Hg2+, Li+) and 14 anions (SO42-, Ac-, NO2-, NO3-, F-, CO32-, HCO3-, H2PO4-, HPO42-, H4BO4-, OH-, Cl-, I-, Br-) were tested. The responses (F/F1) of the assay against Ag+ (600 nmol/L) and other ions (6 µmol/L) were described (Fig. 5). As expected, Ag+ led to an evident increase in the F/F1 values. It is worth mentioning that, none of other ions could cause the signal response even Hg2+. As one of the most toxic metal ions, Hg2+ is easily bound with biomolecules, hence contributing to the interference and difficulty in biosensing. Yet, a slight response from Hg2+ manifested the validity of Ag+-enhanced fluorescence of peptide-AuNPs complexes in theory and practice. Furthermore, the output signal of the mixture of Ag+ and other ions was similar to that of solely Ag+ ions, emphasizing that tested ions had no interference on the determination of Ag+. In stark sharp contrast to other sensing methods[12, 24], the detection time of which exceeds 30 min, our assay is one of the fastest Ag+ sensors with remarkable sensitivity and selectivity.
 |
Fig. 5 The fluorescence responses (F/F1) of peptide-AuNPs treated with Ag+ (c =600 nmol/L) and several common ions (c =6 µmol/L) (a) Cations; (b) Anions. The error bars represent standard deviations based on three independent measurements. |
2.5 Practical Application
In certain environmental samples, such as lake water, the concentrations of some metal ions or some unknown pollutants are obviously higher than that of Ag+, so practical assay is obligatory, and it is a crucial issue for the application of most common sensors. To stress the potential application of the protocol, we prepared water samples from Donghu Lake (in Wuhan City, Hubei Province, China) and filtered through a 0.45 µm membrane and then collected a series of samples by spiking them with different concentrations of Ag+ (100, 300, 500 nmol/L). As shown in Table 1, the recovery results of Ag+ in lake water samples were presented, declaring great applicability and feasibility of this sensor. Besides that, the approach to determinate the recovery of Ag+ in tap water and drinking water was successfully accomplished. On this basis, we believe that the given peptide-AuNPs can be employed as fluorescent probes for Ag+, which holds great potential in practical applications.
Results of the Ag+ recovery experiments performed in lake water, tap water and drinking water (unit:nmol/L)
3 Conclusion
In brief, we proposed a simple, rapid, unlabeled and extremely selective Ag+ detection platform, from which the addition of Ag+ greatly boost the fluorescence intensity of peptides-AuNPs complexes. The peptides of special sequence have ability to stabilize AuNPs by fast modification, along with their intrinsic fluorescence quenching. Upon the addition of Ag+, peptide-AuNPs could undergo the fluorescence recovery, benefit from the coordination between the peptides and Ag+. The detection limit of this strategy was approximately 2.4 nmol/L with the detecting range 5 to 800 nmol/L, far below the limit (460 nmol/L) defined by the USEPA in drinking water. More importantly, the project maintains high selectivity to Ag+ even in the presence of 31 common ions at high concentrations, completely eliminating the disturbance of Hg2+, Pb2+ in biosensing. All analyses could be accomplished within 10 min. Taken the superiorities into consideration, such as simplicity, rapidness of the detecting process, superior sensitivity, and selectivity, great stability of peptide-AuNPs complexes, our method is advantageous over other colorimetric approaches and prospective for on-site rapid monitoring of Ag+.
It can be predicted that future efforts will focus on the development of functional metal-peptide sensing systems. And the platform involving the behavior of metal ions and peptides might remain high attraction for further investigation in the foreseeable future, as they present diverse challenges in the structure, reactivity, mechanism, and synthesis.
References
- Ratte H T. Bioaccumulation and toxicity of silver compounds: A review[J]. Environmental Toxicology and Chemistry, 1999, 18(1): 89-108. [Google Scholar]
- Ceresa A, Radu A, Peper S, et al. Rational design of potentiometric trace level ion sensors. A Ag+-selective electrode with a 100 ppt detection limit[J]. Analytical Chemistry, 2002, 74(16): 4027-4036. [Google Scholar]
- Huang S S, He S, Lu Y, et al. Highly sensitive and selective fluorescent chemosensor for Ag+ based on a coumarin-Se2N chelating conjugate[J]. Chemical Communications, 2011, 47(8): 2408-2410. [Google Scholar]
- Bianco C, Kezic S, Crosera M, et al. In vitro percutaneous penetration and characterization of silver from silver-containing textiles[J]. International Journal of Nanomedicine, 2015, 10: 1899-1908. [Google Scholar]
- Han F X, Patterson W D, Xia Y J, et al. Rapid determination of mercury in plant and soil samples using inductively coupled plasma atomic emission spectroscopy, a comparative study[J]. Water, Air, and Soil Pollution, 2006, 170(1): 161-171. [Google Scholar]
- Yuan J J, Xie Y Z, Han C, et al. Determination of trace element silver in animal serum, tissues and organs by microwave digestion-ICP-MS[J]. Spectroscopy and Spectral Analysis, 2014, 34(9): 2533-2537(Ch). [Google Scholar]
- Guo W, Hu S H, Zhang J Y, et al. Elimination of oxide interferences and determination of ultra-trace silver in soils by ICP-MS with ion-molecule reactions[J]. Science of the Total Environment, 2011, 409(15): 2981-2986. [Google Scholar]
- Li C, Numata M, Takeuchi M, et al. A sensitive colorimetric and fluorescent probe based on a polythiophene derivative for the detection of ATP[J]. Angewandte Chemie (International Edition), 2005, 44(39): 6371-6374. [Google Scholar]
- Mirkin C A, Letsinger R L, Mucic R C, et al. A DNA-based method for rationally assembling nanoparticles into macroscopic materials[J]. Nature, 1996, 382(6592): 607-609. [CrossRef] [PubMed] [Google Scholar]
- Lin C Y, Yu C J, Lin Y H, et al. Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of Tween 20-stabilized gold nanoparticles[J]. Analytical Chemistry, 2010, 82(16): 6830-6837. [Google Scholar]
- Wang G Q, Chen Z P, Chen L X. Mesoporous silica-coated gold nanorods: Towards sensitive colorimetric sensing of ascorbic acid via target-induced silver overcoating[J]. Nanoscale, 2011, 3(4): 1756-1759. [Google Scholar]
- Yang X L, Wei W, Jiang J H, et al. Conformational switching of G-quadruplexes as a label-free platform for the fluorescence detection of Ag+ and biothiols[J]. Analytical Methods, 2016, 8(2): 311-315. [Google Scholar]
- Lin Z Z, Li X H, Kraatz H B. Impedimetric immobilized DNA-based sensor for simultaneous detection of Pb2+, Ag+, and Hg2+[J]. Analytical Chemistry, 2011, 83(17): 6896-6901. [Google Scholar]
- Zhang J F, Lim C S, Cho B R, et al. A two-photon excited luminescence of water-soluble rhodamine-platinum(II) complex: Fluorescent probe specific for Hg2+ detection in live cell[J]. Talanta, 2010, 83(2): 658-662. [Google Scholar]
- Zhang J F, Kim J S. Small-molecule fluorescent chemosensors for Hg2+ ion[J]. Analytical Sciences, 2009, 25(11): 1271-1281. [Google Scholar]
- Ono A, Cao S Q, Togashi H, et al. Specific interactions between silver(I) ions and cytosine-cytosine pairs in DNA duplexes[J]. Chemical Communications, 2008(39): 4825-4827. [CrossRef] [PubMed] [Google Scholar]
- Torigoe H, Kozasa T, Ono A. Detection of C: C mismatch base pair by fluorescence spectral change upon addition of silver (I) cation: Toward the efficient analyses of single nucleotide polymorphism[J]. Nucleic Acids Symposium Series, 2006, 50(1): 89-90. [Google Scholar]
- Wu Z T, Liu Y F, Liu Y Z, et al. A simple and universal "turn-on" detection platform for proteases based on surface enhanced Raman scattering (SERS)[J]. Biosensors and Bioelectronics, 2015, 65: 375-381. [Google Scholar]
- Li X Y, Wu Z T, Zhou X D, et al. Colorimetric response of peptide modified gold nanoparticles: An original assay for ultrasensitive silver detection[J]. Biosensors and Bioelectronics, 2017, 92: 496-501. [Google Scholar]
- Li B, Li K K, Xu W, et al. Micro-interfaces modulation by UV: Ozone substrate treatment for MPEA vapor fluorescence detection[J]. Nano Research, 2023, 16(3): 4055-4060. [Google Scholar]
- Chen P, Shan G G, Nie Q L, et al. Two-color emissive AIEgens with anti-Kasha property for dual-organelle imaging and phototherapy[J]. Science China Chemistry, 2024, 67(5): 1740-1752. [Google Scholar]
- He A X, Xia F F, Han D, et al. Self-assembled amphiphilic NIR-II emissive nano-micelles for imaging-guided photothermal therapy of colorectal cancer[J]. Science China Chemistry, 2024, 67(8): 2767-2774. [Google Scholar]
- Chai F, Wang C G, Wang T T, et al. Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles[J]. ACS Applied Materials & Interfaces, 2010, 2(5): 1466-1470. [Google Scholar]
- Guo J H, Kong D M, Shen H X. Design of a fluorescent DNA IMPLICATION logic gate and detection of Ag+ and cysteine with triphenylmethane dye/G-quadruplex complexes[J]. Biosensors and Bioelectronics, 2010, 26(2): 327-332. [Google Scholar]
- Lee V W, Li H B, Lau T C, et al. Relative silver(I) ion binding energies of α-amino acids: A determination by means of the kinetic method[J]. Journal of the American Society for Mass Spectrometry, 1998, 9(8): 760-766. [Google Scholar]
- Forbes M W, Bush M F, Polfer N C, et al. Infrared spectroscopy of arginine cation complexes: Direct observation of gas-phase zwitterions[J]. J Phys Chem A, 2007, 111(46): 11759-11770. [Google Scholar]
- Remko M, Fitz D, Rode B M. Effect of metal ions (Li+, Na+, K+, Mg2+, Ca2+, Ni2+, Cu2+ and Zn2+) and water coordination on the structure and properties of l-histidine and zwitterionic l-histidine[J]. Amino Acids, 2010, 39(5): 1309-1319. [Google Scholar]
- Hong G S, Tabakman S M, Welsher K, et al. Metal-enhanced fluorescence of carbon nanotubes[J]. Journal of the American Chemical Society, 2010, 132(45): 15920-15923. [Google Scholar]
- Aslan K, Wu M, Lakowicz J R, et al. Fluorescent core-shell Ag@SiO2 nanocomposites for metal-enhanced fluorescence and single nanoparticle sensing platforms[J]. Journal of the American Chemical Society, 2007, 129(6): 1524-1525. [Google Scholar]
- Dong J, Zhang Z L, Zheng H R, et al. Recent progress on plasmon-enhanced fluorescence[J]. Nanophotonics, 2015, 4(1): 472-490. [Google Scholar]
- Bauch M, Toma K, Toma M, et al. Plasmon-enhanced fluorescence biosensors: A review[J]. Plasmonics, 2014, 9(4): 781-799. [Google Scholar]
All Tables
Results of the Ag+ recovery experiments performed in lake water, tap water and drinking water (unit:nmol/L)
All Figures
 |
Fig. 1 Schematic illustrations of Ag+-enhanced fluorescence of peptide-AuNPs |
| In the text | |
 |
Fig. 2 UV-vis spectra of AuNPs and fluorescence emission spectra of peptides RFPRGGDD |
| In the text | |
 |
Fig. 3 Effects of AuNPs size (a), reaction time (b), pH(c), PB concentration (d) on quenching fluorescence of peptides |
| In the text | |
 |
Fig. 4 Ag+ sensing performance of the peptide-AuNPs system (a) Fluorescence spectra of peptide-AuNPs with different Ag+ concentra-tions; (b) Responses (F/F1) of peptide-AuNPs with different Ag+ con-centrations, and inset shows the response linearity of the assay at the Ag+ concentrations range of 5 to 800 nmol/L; (c) Reaction time of peptide-AuNPs with Ag+. |
| In the text | |
 |
Fig. 5 The fluorescence responses (F/F1) of peptide-AuNPs treated with Ag+ (c =600 nmol/L) and several common ions (c =6 µmol/L) (a) Cations; (b) Anions. The error bars represent standard deviations based on three independent measurements. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


