| Issue |
Wuhan Univ. J. Nat. Sci.
Volume 26, Number 6, December 2021
|
|
|---|---|---|
| Page(s) | 521 - 526 | |
| DOI | https://doi.org/10.1051/wujns/2021266521 | |
| Published online | 17 December 2021 | |
Biology
CLC number: R694
Can Hybrid Synapse be Formed between Rat Spinal Motor Neurons and Major Pelvic Ganglion Neurons in vitro?
Department of Surgery, Maternal and Child Health Hospital of Hubei Province, Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430070, Hubei, China
Received:
23
August
2021
The purpose of this study is to determine whether synapses can be formed between spinal motor neurons (SMNs) and major pelvic ganglion (MPG) neurons of a rat in vitro. The green fluorescent protein (GFP)-labelled MPG cells were cultured together with SMNs in a specific medium. The synaptic-like contacts established between SMNs and MPG neurons were studied in co-cultures using morphologic and immunocytochemistry approaches. Phase-contrast observation of co-cultures showed apparent SMNs-MPG neurons contacts as early as three or four days in vitro. We demonstrate some evidence of synaptic contacts between SMNs and MPG neurons in vitro by immunostaining with antibody directed against postsynaptic density protein 95 (PSD-95). We describe the development process of a defined SMNs-MPG neurons co-culture system. The results suggest that the hybrid synapse formation that may occur between SMNs and MPG neurons in vitro played an essential role in the mechanisms of a regenerated bladder with an artificial somatic-autonomic reflex arc.
Key words: spinal motor neurons / major pelvic ganglion / co-culture / synapse
Biography: CHENG Shigang, male, Associate chief physician, M.D., research direction: pediatric urology. E-mail: chenggang527@163.com
Foundation item: Supported by Key Natural Science Foundation Project of Hubei Province (2013CFA069)
© Wuhan University 2021
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
0 Introduction
There has been evidence that indicates synaptogenesis can occur between somatic and autonomic neurons in vivo. Previous studies show that functional synapses have been formed in mammalian sympathetic ganglia re-innervated with somatic nerves[1] . The formation of functional hybrid synapses has been observed in a frog heart when the proximal hypoglossal nerve was anastomosed with the distal vagus nerve[2-4]. It has been shown that stimulation of the redirected hypoglossal nerve could produce a parasympathetic-like cardiac inhibition in the absence of vagal regeneration. Shafik[5] demonstrated that re-innervation of a denervated rectum using a somatic nerve implant is possible in dogs.
Hybrid synapses can be formed in vitro. Nurse et al[6] described the formation of cholinergic synapses between dissociated sympathetic neurons and skeletal myotubes of the rat in cell culture. Belenky et al[7] developed a system for long-term co-culturing of explants of sympathetic chain ganglia and dorsal root ganglia from newborn rats. They found that some apparently specific sympathetic-sensory contacts could be observed, suggesting that a functional interaction may develop between sympathetic axons and sensory neurons in vitro.
Xiao[8] established an artificial somatic-autonomic reflex pathway to restore controllable micturition after spinal cord injury. Xiao’s procedure, however, assumed that motor axons were able to regenerate into autonomic preganglionic nerves, re-innervate the parasympathetic ganglion cells of the bladder and thus transfer somatic reflex arc activity to the bladder’s smooth muscle[9,10]. Owing to the complexity involved in studying a neuron coupled to its target neuron in vivo, mechanisms underlying hybrid synapse formation are poorly understood. The obvious advantage of in vitro systems is that they are much simpler and therefore more straightforward in elucidating the mechanisms underlying hybrid synapse formation. The complexity of the innervation patterns of somatic and autonomic neurons in situ lead us to question whether somatic-autonomic interactions can be effectively modeled by an in vitro co-culture system.
To explore whether synapses would form between spinal motor neurons (SMNs) and major pelvic ganglion (MPG) neurons of the rat, we developed a system for the co-culture of SMNs from a newborn rat with MPG neurons from an adult rat. This study would allow for better understanding of the underlying mechanisms of the hybrid somatic-autonomic synapse.
1 Materials and Methods
1.1 Animals and Main Reagents
All animal experiments were approved by the Institutional Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology. All efforts were made to minimize animal suffering or discomfort and the number of animals used. Recombinant serotype 5 adenovirus (Ad5) expressing enhanced green fluorescent protein (Ad-GFP) under the CMV promoter was purchased from Chinese National Human Genome Center, Beijing, China. Mouse monoclonal anti-neurofilament 200 (NF-200) was from Sigma, USA. Rabbit polyclonal choline acetyl transferase (ChAT) was from Boster, Wuhan, China. Rabbit polyclonal anti-PSD-95 was from Invitrogen, USA.
1.2 Culture of MPG Neurons
MPGs closely attached to the ventral part of the prostate[11] were dissected from adult male Sprague-Dawley rats (3-6 months). Animals were anesthetized with chloralhydrate (Sigma) as previously described[12]. The major pelvic ganglions were removed and neurons were dissociated and cultured under serum-free conditions, as previously described[13]. In brief, Ganglions were first digested with collagease (1 mg/mL, Sigma) for 30 min at 37 ℃ in neurobasal media (Gibco, Neurobasal® Medium) with gentle agitation for 10 min. After being washed twice with D-Hanks solution, ganglions were digested with trypsin (1 mg/mL, Sigma). Slices were triturated about 10 times using a fire-polished glass pipette. The solution was allowed to settle for 2 min and the supernatant was then transferred to another tube. Fresh medium was added with further trituration performed, then the cells were removed again. This sequential trituration was repeated until all slices were nearly or totally dissociated. Neurons were plated onto glass coverslips that had been pre-coated overnight with 50 μg/mL poly-L-lysine (Sigma) at about 0.6×104 neurons per mL. Neurons were cultured in Neurobasal medium containing 2% B27 (Invitrogen), 1% penicillin/ streptomycin, 20 ng/mL NGF.
1.3 GFP Expression
The cultures were maintained for 2 days, after which MPG cells were transfected with Ad-GFP and placed in a 37 ℃/5% CO2 incubator for 24 h. For the infection of the MPG neurons, multiplicity of infection (MOI) at 300, 500, 800 and 1 000 were used. Starting from a day after the viral infection, the expression of GFP was examined for a total of 2 weeks under fluorescent microscopy.
1.4 Culture of SMNs
The newborn rat’s lumbar spinal cord was exposed under a dissecting microscope and the dorsal aspect of the vertebral column removed quickly using curved jeweler’s forceps. The slices were then digested in pre-warmed trypsin by a modification of the method described previously[14,15]. The slices were briefly digested in pre-warmed trypsin at 37 ℃ for 30 min by frequent agitation. The tissues were then gently triturated using sterile fire-polished glass pipettes until the cells dispersed into a single cell suspension. After the pieces were left to settle, the supernatant was centrifuged through a layer of 6.7% metrizamide and spun for 15 min at 3 000 r/min. The interface medium was collected and the fragments containing cells were re-suspended again gently. The dissociated cell suspension was allowed to sediment for 30 min. The SMNs were gently re-suspended and diluted to the concentration at a density of 6×104 cells/mL.
1.5 Co-Culture of SMNs and MPG Neurons
The medium bathing the MPG neurons was completely removed and replaced with 1 mL of medium containing SMNs. On day 2 of the co-culture, the medium was fully replaced with a defined serum-free medium and the cells were maintained at 37 ℃ in a 5% CO2 incubator. After that, half of the medium was replaced every 2 or 3 days and the system provided a maximum survival of more than 2 weeks. Both the GFP expression of adult MPG neurons and the cell growth of co-culture system were observed under an Olympus TH4-200 Fluorescence Microscope.
1.6 Immunocytochemical Staining
Mouse monoclonal anti-neurofilament 200 (NF-200) was used to morphologically identify MPG neurons. Rabbit polyclonal choline acetyl transferase (ChAT), a marker of motor neurons, was performed to identify the SMNs. Immunocytochemical evidence for synapse formation between SMNs and MPG neurons was shown by postsynaptic density protein 95 (PSD-95). For the detection of PSD-95 immunoreactivity, following 12 days of co-culture in vitro, neurons were fixed for 20 min in PBS containing 4% paraformaldehyde at room temperature. After rinsing in PBS, cells were permeabilized with 5% BSA for 20 min at room temperature and non-specific sites were blocked. Cultures were incubated in rat anti-PSD-95 primary antibody (1∶250) overnight at 4 ℃ in a humidified chamber. After being washed three times in PBS, cells were incubated for 20 min at 37 ℃ in cy3- labeled goat anti-rat IgG (1:100). Photographs were viewed by an Olympus TH4-200 Fluorescence Microscope and laser scanning confocal microscope.
2 Results
2.1 Determination of SMNs and MPG Neurons
In our studies, SMNs and MPG neurons were determined by fluorescent labeling. SMNs were identified by staining with ChAT. MPG neurons were identified by immunostaining with antibody directed against NF-200. Staining with the antibody revealed the entire extent of the neuron and its processes (Fig. 1).
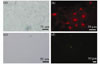 |
Fig. 1 Characteristics of spinal motor neurons ((a), (b)) and major pelvic ganglion neurons ((c), (d)) Spinal motor neurons were stained with the ChAT antibody (b), axon-like processes could be identified; Cultured major pelvic ganglion neurons identified by fluorescent labelling of NF-200 (d), which defined the cell soma and extensive neuritic processes |
2.2 GFP Expression in MPG Neurons
Embryonic neurons represent an easy and effective source of primary neurons. However, the anatomical location of MPG in the embryonic rat was difficult to precisely identify. In our later experiments, MPG neurons were dissected from adult rats. After 2 days in vitro, we observed that MPG neurons were able to attach efficiently. The somas were normally ovoid or fusiform but not exclusively so. Some of the neurons in culture were associated with several primary neuritic processes, secondary and tertiary branches that were observed as well. Cells were analyzed for expression 48 h after virus infection. The results showed that the GFP expression in Ad-GFP transduced neurons could be visualized directly under fluorescence microscope (Fig. 2). The efficiency of infection was expressed as the percentage of the total cell numbers expressing bright green fluorescence. With an MOI of 500-800, after 48 h, about 80% MPG neurons expressed GFP that revealed the entire extent of the cell soma and processes. Most importantly, we did not observe any toxicity even with high MOI. With the MOI of 1 000, the infected neurons displayed bright green fluorescence, but their morphologies changed drastically and a large number of MPG neurons died quickly. After MPG cells were transfected with Ad-GFP vector, the intensity of fluorescence gradually increased over time in each GFP-positive cell. The expression of GFP was at its peak from the 7th day to the 14th day. Intensity gradually decreased after 14 days.
 |
Fig. 2 Adult major pelvic ganglion neurons labeled by GFP gene recombinant adenovirus Dissociated neurons were infected with the GFP recombinant adenovirus at different cultures. GFP was presented throughout the infected cells, including the cell soma and processes. (a) 24 h; (b) 8 d |
2.3 Establishment of an in vitro Co-Culture System
Starting from a day after MPG cells were transfected with Ad-GFP vector, SMNs were plated at a density of 6×104 cells/mL on the top of the MPG neurons. The GFP expressed in MPG neurons facilitated the observation of the interaction without requiring immunostaining. After 3-4 days in co-culture, some GFP-labeled MPG neurons and GFP-negative cells were visualized in proximity with each other. With time in the co-culture, MPG neurons gradually matured and emitted outgrowing processes of which some came into association with GFP-negative cells (Fig. 3). Through combining phase contrast and fluorescent microscopy, we observed several instances that GFP-labeled processes came close to soma of the SMNs. Some SMN-MPG neuron contacts suggested that a functional interaction may develop between somatic-autonomic neurons in vitro.
 |
Fig. 3 Characterization of major pelvic ganglion neurons and spinal motor neurons in co-culture (a), (b): Phase contrast images of a major pelvic ganglion neuron contacted by multiple spinal motor neurons. (c), (d): GFP-labelled major pelvic ganglion neurons and spinal motor neurons were shown in proximity to each other. Some apparent SMN-MPG neuron contacts are seen in (a), (b) and (d) (arrows) |
2.4 Immunocytochemical Staining Result
Immunocytochemical evidence for synapse-like formation between the SMNs and MPG neurons was shown by the close proximity of postsynaptic marker, PSD-95. In the co-culture, the plating density of SMNs was 10-fold that of the MPG neurons, so the MPG neurons were scattered among the SMNs. Both SMNs and MPG neurons could be stained by the PSD-95 antibody. We observed red dot clusters that were mainly located at soma or proximal neurite. After characterizing the population of MPG neurons in our culture system by expressing GFP, we examined the temporal pattern of synapse-like formation between these SMNs and MPG neurons. To do this, we assessed the localization of a postsynaptic marker. To selectively identify postsynaptic terminals of MPG neurons, staining with PSD95 was performed (Fig. 4). Using this staining approach, we evaluated the co-localization of MPG postsynaptic PSD clusters (red) with GFP (green). Because MPG neurons were scattered among SMNs in our co-culture system, we assumed that synapse formation was between SMNs and MPG neurons. We observed that yellow puncta were preferentially localized at the site where the dendrite of a SMN contacted the soma of a MPG neuron. This suggested that the neurons formed synaptic contact at these points.
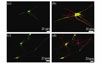 |
Fig. 4 Immunocytochemical evidence for synapse formation in co-cultures (a), (c): MPG neurons were labeled with GFP; (b), (d): Confocal image of major pelvic ganglion neurons expressing GFP and spinal motor neurons co-cultures. A high density of brightly stained puncta of PSD (red) immunoreactivity was observed outlining the GFP-positive cell bodies and dendrites |
3 Discussion
In this paper, we have demonstrated the development of a defined SMNs-MPG neurons co-culture system. Phase-contrast observation of co-cultures shows apparent neuro-neuronal contacts after only 3 DIV. We observed that our culture conditions in serum-free medium have allowed the formation of synapses between somatic and autonomic neurons.
Our knowledge of the molecular organization and development of hybrid synapses is sparse due to the lack of a reliable model system that reconstitutes synaptogenesis between these two neuronal populations. Investigations suggest that the area of tissue culture offers opportunities for research. It is well established that the co-culture technique can be used to study synaptogenesis between cells derived from different regions. The technical difficulties in studying individual neurons in situ can be overcome with the development of in vitro approaches. Zhou et al[16,17] reported that co-culture with primary neural cells was helpful for PC12 cells’ differentiation and their neurite growth induced by NGF. These PC12 derived neuron-like cells could form functional synapses with primary neurons. Rene et al[18] studied the establishment of synaptic contacts between GABAergic or dopaminergic neurons and the melanotrope cell by morphofunctional approaches. Kentaro et al[19] reported on the formation of functional synapses between the olfactory receptor neurons in the epithelium and the neurons in the olfactory bulb.
The adenovirus is currently an efficient and safe vector for nerve system gene delivery as it infects non-replicating neurons and does not cause insertional mutagenesis of host cell genomes. GFP has been shown to have characteristics that make it ideal for use as an expression marker in living cells[20]. We observed that the infection efficiency was up to 80% after 48 h when MOI was 500-800. The infected MPG neurons were vigorously growing for 2 weeks.
As early as after 2 days DIV, SMNs extended neurites, formed homotypic contacts and established a dense neuritic network. Phase-contrast observation of co-cultures showed apparent neuro-neuronal contacts after 3-4 days DIV. SMNs emitted numerous outgrowing neurites of which some came into association with MPG neurons in co-culture. We also found that with time, the number of MPG neurons which came close to SMNs increased. The establishment of these SMNs-MPG neurons contacts is subsequent to the formation of the SMNs-SMNs contacts. The delay observed in the formation of SMNs- MPG neurons than that of SMNs-SMNs contacts might be due to the lower density of MPG neurons compared with that of SMNs neurons (1:10). This delay may also be related to the need of specific factors from MPG neurons, such as neurotrophic factors or cell surface molecules which might require some time in culture after dissociation prior to their functioning.
PSD-95, the postsynaptic marker, is used to assess the localization of synaptic protein into postsynaptic structures[21,22]. We used an antibody PSD-95 to quantify synaptogenesis. We demonstrated morphological contacts between SMNs and MPG neurons as early as 3 days in vitro. After 12 DIV, some of GFP-positive neurons were labeled with the PSD antibody. Co-localization of PSD-95 with GFP revealed that the PSD-95-immunopositive cells were MPG neurons. Immunoreactivity was distributed diffusely in the cell body and in the proximal part of neuritis. Progressively, the number of SMNs-MPG neurons contacts increased with more time in the culture.
4 Conclusion
In this study, we have described a model of co-culture which appears well adapted to analyze the mechanisms of synapse formation between pairs of connected neurons. Our data has suggested that the hybrid synapse formation may occurred between SMNs and MPG neurons in vitro. An in vitro co-culture system would be useful for studies on factors regulating development of somatic-autonomic synapses. The hybrid synapse formation between SMNs and MPG neurons was examined in terms of its morphology and immunocytochemistry. But direct synapse-like contacts between SMNs-MPG neurons should be observed by electron microscopy. We should attempt to record electrophysiologically from SMNs-MPG neurons co-cultures.
Ethical Statement
All applicable national and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
- McLachlan E M. The formation of synapses in mammalian sympathetic ganglia reinnervated with preganglionic or somatic nerves [J]. J Physiol, 1974, 237(1): 217-242. [Google Scholar]
- Proctor W, Frenk S, Taylor B, et al. “Hybrid” synapses formed by foreign innervation of parasympathetic neurons: A model for selectivity during competitive reinnervation [J]. Proc Natl Acad Sci, 1979, 76(9): 4695-4699. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Proctor W, Roper S. Competitive elimination of foreign motor innervation on autonomic neurones in the frog heart [J]. J Physiol, 1982, 326: 189-200. [Google Scholar]
- Proctor W, Roper S, Taylor B. Somatic motor axons can innervate autonomic neurones in the frog heart [J].J Physiol, 1982, 326: 173-188. [Google Scholar]
- Shafik A. Reinnervation of the rectum with a somatic nerve: A canine study [J]. Spinal Cord, 1997, 35(1): 53-57. [CrossRef] [PubMed] [Google Scholar]
- Nurse C A, O’Lague P H. Formation of cholinergic synapses between dissociated sympathetic neurons and skeletal myotubes of the rat in cell culture [J]. Proc Natl Acad Sci, 1975, 72(5): 1955-1959. [Google Scholar]
- Belenky M, Devor M. Association of postganglionic sympathetic neurons with primary afferents in sympathetic-sensory co-cultures [J]. J Neurocytol, 1997, 26(11): 715-731. [Google Scholar]
- Xiao C G. Reinnervation for neurogenic bladder: Historic review and introduction of a somatic-autonomic reflex pathway procedure for patients with spinal cord injury or spina bifida [J]. Eur Urol, 2006, 49(1): 22-28. [Google Scholar]
- Xiao C G, Du M X, Dai C, et al. An artificial somatic-central nervous system-autonomic reflex pathway for controllable micturition after spinal cord injury: Preliminary results in 15 patients [J]. J Urol, 2003, 170(4 Pt 1): 1237-1241. [CrossRef] [PubMed] [Google Scholar]
- Xiao C G, Du M X, Li B, et al. An artificial somatic-autonomic reflex pathway procedure for bladder control in children with spina bifida [J]. J Urol, 2005, 173(6): 2112-2116. [Google Scholar]
- Dail W G, Evan A P, Eason H R. The major ganglion in the pelvic plexus of the male rat: A histochemical and ultrastructural study [J]. Cell Tiss Res, 1975, 159: 49-62. [Google Scholar]
- Steers W D, Kolbeck S, Creedon D, et al. Nerve Growth factor in the urinary bladder of the adult regulates neuronal form and function [J]. J Clin Inest, 1991, 88: 1709-1715. [Google Scholar]
- Cheng S, Yang X, Zhang Y, et al. Culture of major pelvic ganglion neurons from adult rat [J]. Cytotechnology, 2013, 65(4): 663-669. [CrossRef] [PubMed] [Google Scholar]
- Cheng S, Shi Y, Hai B, et al. Culture of motor neurons from newborn rat spinal cord [J]. J Huazhong Univ Sci Technolog Med Sci, 2009, 29(4): 413-416. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Ullian E M, Harris B T, Wu A, et al. Schwann cells and astrocytes induce synapse formation by spinal motor neurons in culture [J]. Mol Cell Neurosci, 2004, 25(2): 241-251. [CrossRef] [PubMed] [Google Scholar]
- Zhou T, Xu B, Chen D, et al. The formation of functional synapse in between neurons differentiated from PC12 cells and the primary neurons cultivated in vitro from neonatal cortex of rats [J]. Chinese Journal of Neurology, 2005, 38(3): 183-186. [Google Scholar]
- Zhou T, Xu B, Que H, et al. Neuronal PC12 cells develop functional synapses with neurons from neonatal cortex (a FM1-43 imaging investigation) [J]. Chinese Journal of Neurosurgery, 2005, 21(3): 182-186. [Google Scholar]
- Rene F, Poisbeau P, Egles C, et al. Co-culture of hypothalamic neurons and melanotrope cells: A model to study synapses between central neurons and endocrine cells [J]. Neuroscience, 1997, 76(1): 203-214. [Google Scholar]
- Kentaro K, Kimihiko S, Makoto K. Functional synapse formation between rat olfactory receptor neurons and olfactory bulb neurons in vitro [J]. Neuroscience Letters, 2000, 285: 76-78. [CrossRef] [PubMed] [Google Scholar]
- Sarkar P, Chattopadhyay A. GFP fluorescence: A few lesser-known nuggets that make it work [J]. J Biosci, 2018, 43(3): 421-430. [Google Scholar]
- Savioz A, Leuba G, Vallet P G. A framework to understand the variations of PSD-95 expression in brain aging and in Alzheimer’s disease [J]. Ageing Res Rev, 2014, 18: 86-94. [Google Scholar]
- Chen X, Winters C, Crocker V, et al. Identification of PSD-95 in the postsynaptic density using miniSOG and EM tomography [J]. Front Neuroanat, 2018, 12: 107. [Google Scholar]
All Figures
 |
Fig. 1 Characteristics of spinal motor neurons ((a), (b)) and major pelvic ganglion neurons ((c), (d)) Spinal motor neurons were stained with the ChAT antibody (b), axon-like processes could be identified; Cultured major pelvic ganglion neurons identified by fluorescent labelling of NF-200 (d), which defined the cell soma and extensive neuritic processes |
| In the text | |
 |
Fig. 2 Adult major pelvic ganglion neurons labeled by GFP gene recombinant adenovirus Dissociated neurons were infected with the GFP recombinant adenovirus at different cultures. GFP was presented throughout the infected cells, including the cell soma and processes. (a) 24 h; (b) 8 d |
| In the text | |
 |
Fig. 3 Characterization of major pelvic ganglion neurons and spinal motor neurons in co-culture (a), (b): Phase contrast images of a major pelvic ganglion neuron contacted by multiple spinal motor neurons. (c), (d): GFP-labelled major pelvic ganglion neurons and spinal motor neurons were shown in proximity to each other. Some apparent SMN-MPG neuron contacts are seen in (a), (b) and (d) (arrows) |
| In the text | |
 |
Fig. 4 Immunocytochemical evidence for synapse formation in co-cultures (a), (c): MPG neurons were labeled with GFP; (b), (d): Confocal image of major pelvic ganglion neurons expressing GFP and spinal motor neurons co-cultures. A high density of brightly stained puncta of PSD (red) immunoreactivity was observed outlining the GFP-positive cell bodies and dendrites |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


