| Issue |
Wuhan Univ. J. Nat. Sci.
Volume 27, Number 4, August 2022
|
|
|---|---|---|
| Page(s) | 349 - 360 | |
| DOI | https://doi.org/10.1051/wujns/2022274349 | |
| Published online | 26 September 2022 | |
Biomedicine
CLC number: R 285
Mechanisms of the Traditional Chinese Herb Atractylodes lancea against COVID-19 Based on Network Pharmacology and Molecular Docking
1
Renmin Hospital, Wuhan University, Wuhan 430060, Hubei, China
2
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education/School of Pharmaceutical Sciences, Wuhan University, Wuhan 430071, Hubei, China
Received:
15
April
2022
Atractylodes lancea (Thunb.) DC. (AL) has been proven to be effective in the treatment of coronavirus disease 2019 (COVID-19). In this study, TCMSP, TCMID, OMIM, GeneCards, PharmMapper and SwissTargetPrediction were used to collect potential targets for AL against COVID-19. The online STRING analysis platform and Cytoscape were used for generating a (protein-protein interaction) PPI network. The Cytoscape and Autodock software were used for determining hub genes and key compounds. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed via DAVID database. A total of 84 common targets were obtained. The antiviral pathways were main pathways in traetment. 10 hub genes and key compounds were screened by CytoHubba. We found that AL2, AL6 and AL38 had lower binding energy with key proteins. Our study demonstrated that AL might be used to treat COVID-19 by improving the "cytokine storm", regulating some antiviral pathways, and inhibiting the key protein through which the SARS-CoV-2 invades the host cell. These findings give a pharmacological basis and support for treating COVID-19 with AL.
Key words: Atractylodes lancea / COVID-19 / mechanism / network pharmacology / molecular docking
Biography: LEI Jiachuan, male, Ph.D., Associate professor, research direction: medicine chemistry and pharmacology. E-mail: leijc@126.com
© Wuhan University 2022
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
0 Introduction
On Jan 7, 2020, Chinese scientists had isolated a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In February, 2020, WHO designated COVID-19[1, 2]. As of February 2022, there were over 410 million confirmed cases and more than 5.8 million deaths worldwide (https://www.worldometers.info/coronavirus/). Food and Drug Administration (FDA) approved the first COVID-19 treatment drug remdesivir in 2020, and announced the benefits of remdesivir outweigh the risks. In addition, favipiravir was reported to have potent antiviral activity in COVID-19 at high doses[3]. Finding drugs through key targets is a fast way, which can be seen from previous studies of similar coronaviruses like SARS-CoV and MERS-CoV. The spike protein, 3CL protease (3CLpro), and angiotensin converting enzyme 2 (ACE2) have been prioritized as SARS-CoV-2 antiviral drug targets[4, 5]. Latest research found that Neuropilin-1 (NRP1) was involved in SARS-CoV-2 infection[6].
Atractylodes lancea (Thunb.) DC. (AL) has been used as a traditional Chinese medicine (TCM) for thousands of years. The rhizome of AL is used extensively for the treatment of several diseases such as rheumatic diseases, digestive disorders, and influenza[7]. In recent years, some researchers have found that AL and related prescriptions have inhibitory effect on human rotavirus, viruses H3N2 and Influenza A virus[8-10]. The prescriptions containing AL, such as Qingfei Paidu decoction and Huashi Baidu decoction, are effective prescriptions based on clinical observations in the early clinical treatment of COVID-19.
AL has a significant therapeutic effect on COVID-19, but the mechanisms and the key compounds are still unclear. With the development of bioinformatics and network pharmacology, the network-based approach to drug discovery is seen as promising for rapid and cost-effective drug development[11]. Network pharmacology was defined as pharmacology that the "multi-component, multi-target, multi-pathway" synergistic relationship between drugs, diseases and targets was analyzed by network methods[12]. TCM is a complex system and follows a holistic approach to disease treatment, and network pharmacology builds a bridge to study the relationship between TCM and modern pharmacology. Therefore, it is necessary to find the potential mechanisms and key compounds of AL against COVID-19 with network pharmacology.
At present, few studies on AL against COVID-19 mainly focus on preliminary network analysis[13]. In this study, TCMSP, TCMID, OMIM, GeneCards were used for collecting targets, and the Cytoscape software were used for building and analyzing network. On the basis of network pharmacology, we combined molecular docking to verify the analysis results. We hope to find the mechanisms and active compounds of AL against COVID-19.
1 Materials and Methods
1.1 Screening Active Compounds and Targets of AL
The compounds from AL were collected from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform[14] (TCMSP) (https://tcmspw.com/tcmsp.php) and the Traditional Chinese Medicine Integrated Database[15] (TCMID) (http://www.megabionet.org/tcmid/). Then, the pharmacokinetic of all compounds were analyzed by SwissADME[16] (http://www.swissadme.ch/). Later, the active compounds were imported into the PharmMapper Server[17, 18] (http://www.lilab-ecust.cn/pharmmapper/) and SwissTargetPrediction[19, 20] (http://www.swisstargetprediction.ch/) to obtain the targets. There were different scoring criteria among different databases. Targets with Norm Fit value of >0.5 in PharmMapper Server or Probability value of >0 in SwissTargetPrediction were selected as the targets of AL. Then, the gene names were unified by Uniprot (https://www.uniprot.org/). Finally, the predicted targets were collected and the duplications were removed.
1.2 Screening Targets of COVID-19
The disease-related targets were obtained from GeneCards database[21] (https://www.genecards.org/) and Online Mendelian Inheritance in Man (OMIM) database[22] (https://omim.org/) by using "Novel Coronavirus Pneumonia" and "COVID-19" as keywords. Then, the gene names were unified by Uniprot (https://www.uniprot.org/) and the duplications were removed..
1.3 Obtaining Protein-Protein Interaction (PPI) Data
The common targets of AL and COVID-19 were obtained and mapped by Venn diagrams. The protein-protein interaction (PPI) data were obtained from the Search Tool for Recurring Instances of Neighboring Genes (STRING) database[23] (https://string-db.org/), with the species limited to "Homo sapiens". In STRING databases, three confidence score thresholds (0.4, 0.7 and 0.9) were used, and the PPI data with a confidence score of >0.9 was selected to construct the protein interaction network (PIN).
1.4 Network Construction and Analysis
PPI data exported from STRING were imported into Cytoscape 3.6.0 (https://cytoscape.org/), visualized and analyzed after hiding the disconnected nodes. The topology parameter of degree value was determined by the Network-Analyzer tool in Cytoscape software. Furthermore, we adopted the plug-in CytoHubba[24] in Cytoscape software to analyze the data in the PPI network of common targets by the Maximal Clique Centrality (MCC), and found the hub genes and hub compounds. The association between active compounds, common targets and pathways was processed and visualized by Cytoscape software. Then, we used the Molecular Complex Detection (MCODE) algorithm[25] to explore the functional modules in the PIN. MCODE is based on vertex weighting by local neighborhood density and outward traversal from a locally dense seed protein to isolate the dense regions according to the given parameters[11].
1.5 Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
GO and KEGG pathway analyses were performed by the Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8[26] (https://david.ncifcrf.gov/). The visualization of results was performed by Dotchart and Bubble tools in Hiplot (https://hiplot.com.cn).
1.6 Molecular Docking
Molecular docking refers to binding small molecule ligands to macromolecular receptors through computer simulation, and calculating the physical and chemical parameters to predict the binding affinity[27]. The structure files of 3CLpro (PDB:6M2N), ACE2-spike protein complex (PDB:6M0J) and NRP1 (PDB:6FMC) were downloaded from Protein Data Bank (PDB) (http://www.rcsb.org/), and the 3D structure files of ligands were downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/). Before docking, the water molecules on the receptors need to be removed, while polar hydrogen atoms, charge and magnetic field need to be added. We applied AutoDock 4.2 (http://autodock.scripps.edu/) to process ligands and receptors. The binding sites of targets were determined by the information from literatures or small ligands in protein crystals[28]. Then AutoDock Vina 1.1.2 (http://autodock.scripps.edu/) was used for molecular docking and analysis of docking results. The 3D and 2D diagrams of best‑scored binding pose were visualized by Discovery Studio 2020 Client (https://www.neotrident.com/download.html). Through examining the binding mode and binding free energy of the compounds with the corresponding targets, we could determine the affinity between them.
2 Results
2.1 Active Compounds and Targets of AL
The compounds were collected from TCMSP and TCMID, and the GI absorption and drug-likeness were screened by SwissADME. A total of 40 compounds from AL were selected. Among them, one compound was removed due to no target by predicting from SwissTargetPrediction and PharmMapper. Finally, 706 targets of AL were obtained after removing the duplicate values. Compound name and ID were shown in Table 1.
The collected compounds of AL
2.2 PPI Data of AL against COVID-19
We obtained 647 targets of COVID-19 from OMIM and GeneCards databases after removing the duplication. Jvenn[29] (http://bioinfo.genotoul.fr/jvenn) was used to analyze common targets. Then, 84 common targets were obtained, which could be considered as the potential targets (Fig. 1(a)). We imported 84 common targets into the STRING database, and obtained the PPI data including 83 nodes and 1 572 edges (Fig. 1(b)). Next, the PPI data were exported in tab-separated value (TSV) format. Cytoscape software was adopted to analyze and visualize the PIN.
 |
Fig. 1 Identification of the common genes and construction of PPI network (a) Identification of the AL-target and disease-related genes by taking an intersection of AL target genes and COVID-19-related genes; (b) Construction of PPI network of common genes by STRING database |
2.3 MCODE Analysis
Functional modules of PIN were explored by MCODE (Fig. 2). 5 modules were identified with a score of >3, in which we selected 3 modules with a score of >5 for KEGG pathway analysis. The score of Module 1 was 16.211, and the PIN contained 20 nodes and 308 edges (Fig. 2(a)). After KEGG pathway analysis, we found that these targets were mainly enriched in following pathways: chagas disease (American trypanosomiasis), TNF signaling pathway, pertussis, HIF-1 signaling pathway and tuberculosis (Fig. 2(b)). The score of Module 2 was 9.684, and the PIN contains 20 nodes and 184 edges (Fig. 2(c)). The genes of Module 2 were mainly involved in viral carcinogenesis, hepatitis B, pathways in cancer, cell cycle and PI3K-Akt signaling pathway (Fig. 2(d)). Module 3 got the score of 5.333, and the PIN contains 7 nodes and 32 edges (Fig. 2(e)). The genes of Module 3 were mainly involved in colorectal cancer, prostate cancer, T cell receptor signaling pathway, leukocyte transendothelial migration and neurotrophin signaling pathway (Fig. 2(f)).
 |
Fig. 2 The MCODE analysis of PPI network of common genes (a) The genes of module 1 with the score of 16.211; (c) The genes of module 2 with the score of 9.684; (e) The genes of module 3 with the score of 5.333; (b), (d), (f):The KEGG pathway analysis of module 1-3 |
2.4 GO Function and KEGG Pathway Enrichment Analysis
We revealed the biological functions of the common targets by GO enrichment analysis. 421 GO biological function terms were obtained from DAVID database, and slide bead diagram was applied to the top 5 GO terms of biological process (BP), molecular function (MF) and cellular component (CC), respectively (Fig. 3(a)). The results of biological processes analysis indicated that the common targets were significant involved in protein phosphorylation, platelet activation, peptidyl-serine phosphorylation, phosphati-dylinositol-mediated signaling, positive regulation of I-kappaB kinase/NF-kappaB signaling. The results of the cellular component and molecular function analysis showed that the common targets existed in cytoplasm, nucleus, cytosol and extracellular exosome, and their major function at the molecular level are protein binding. To sum up, we speculated the mechanisms of AL against COVID-19 simultaneously involved these biological processes and molecular functions. Then, we analyzed the common targets by DAVID to reveal the information of KEGG pathways. 122 pathways were obtained from KEGG pathways analysis according to the P value, and the top 10 crucially pathways were shown in Fig. 3(b). These pathways included hepatitis B, pathways in cancer, viral carcinogenesis, HIF-1 signaling pathway, influenza A, measles, Epstein-Barr virus infection, hepatitis C, FoxO signaling pathway and pancreatic cancer. These results indicated that 5 antiviral-related pathways were related to the mechanisms of AL against COVID-19, so we speculated that the main way of AL against COVID-19 was controlling the viral infection.
 |
Fig. 3 The visualization of GO and KEGG pathway analysis (a) GO enrichment analysis; (b) KEGG pathway analysis; BP: biological process; MF: molecular function; CC: cellular component |
2.5 PIN of Compound-Target-Pathway (C-T-P)
The correlations among the top 10 KEGG pathways, 84 common targets and active compounds were imported into Cytoscape software to construct the PIN of C-T-P. After removing the values of degree = 0, the PIN was constructed including 133 nodes and 2 457 edges (Fig. 4(a)).
 |
Fig. 4 Compound-Target-Pathway network diagram (a) and hub genes (b) In (a), red rounds represent the 84 common targets of AL against COVID-19, blue squares triangles represent the 39 active compounds of AL, and green triangles represent the 10 key pathways of AL against COVID-19. In (b), lines represent protein-protein interactions present, and the color of rounds from red to yellow represent the score values varying from higher to lower |
2.6 Hub Genes and Key Compounds
CytoHubba was adopted in Cytoscape software to analyze network. By this, 10 hub genes and 10 key compounds were found from PPI network and PIN of C-T-P, respectively. As the score decreases, the color of ten genes (IL6, CASP3, ALB, MAPK1, MAPK8, PTGS2, IL1B, ICAM1, NOS3 and STAT3) changes from red to yellow (Fig. 4(b)), which were considered as crucial genes that played key roles in the treatment of AL against COVID-19. After analyzing and scoring the C-T-P PIN, AL2, AL3, AL6, AL10, AL12, AL24, AL25, AL31, AL36 and AL38 were identified as the key compounds.
2.7 Molecular Docking
We analyzed the binding energy and the mode of interaction of the 10 important compounds and 3 key targets (3CLpro, ACE2-spike complex, NRP1) through molecular docking experiments, and the docking was performed based on active sites. The pocket size of 3CLpro was x =-61.381, y =-34.951, z = 23.145. The pocket size of ACE2-spike complex was x =-33.62, y =-25.001, z= 3.118. The pocket size of NRP1 was x =26.438, y =13.585, z =-13.092.
Remdesivir and favipiravir were used as the control. Almost all the binding energy of 10 important compounds with the key targets was less than -5 kcal/mol (1 kcal=4.1868 kJ) (Table 2).
The modes of the best binding energy of targets were displayed in Fig. 5(a)-(c). The hydrophobic interaction, electrostatic interaction and hydrogen-bond interaction were displayed in 2D results. The space positions, hydrogen bonding and amino acids were displayed in 3D results.
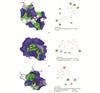 |
Fig. 5 Molecular docking mode of 3 key targets and compounds with lowest binding energy (a) AL6-3CLpro; (b) AL6-NRP1; (c) AL38-ACE2-spike protein complex. Hydrogen bonding was shown by green dashed line |
Results of molecular docking efficiency
3 Discussion
Even to this day, SARS-CoV-2 infection is still spreading in most countries, although many countries have begun vaccination to their people. The outbreak is still not under control. At the same time, mutated viruses like Delta and Omicron began to appear, which greatly increased the difficulty of drug development. TCM has strong effectiveness in treating COVID-19, especially for mild and general cases[30]. In this study, we constructed the PIN of 84 common genes, and screened out 10 hub genes, including IL6, CASP3, ALB, MAPK1, MAPK8, PTGS2, ICAM1, IL1B, NOS3 and STAT3. We found that AL was mainly against COVID-19 through antiviral pathways, and revealed the modules were related to antiviral and immune pathways by MCODE analysis. Furthermore, we also constructed a C-T-P PIN, and screened out 10 key compounds. Finally, we performed molecular docking experiments on key compounds and recognized targets involved in COVID-19, and we obtained the binding energy and the best binding modes. Based on Chinese clinical experience, TCM were effective in the treatment of COVID-19, and we hope to provide some bases for clinical application[31].
Fever, diarrhea and fatigue are the common symptoms of COVID-19[32]. Recent studies had pointed out that "cytokine storms" had appeared in patients with COVID-19. Tumor necrosis factor-α (TNF-α), Interleukin (IL)-1 and IL-6 were recognized as cytokines causing symptoms[33,34]. Our results showed that IL-6 and IL-1β were the hub genes of AL against COVID-19, and the genes of Module 1 were mainly enriched in TNF signaling pathway. It was proved that AL might treat COVID-19 by relieving these symptoms. In addition, "cytokine storm" had been deemed to a primary factor of COVID-19 in severe cases, and persistent "cytokine storm" may cause the serious inflammatory damage of lung tissue[35]. NOS3 may induce nitric oxide production and cause inflammation. In addition, MAPK1 and MAPK8 were recognized as genes closely related to inflammation[36]. Atractylenolide I, Atractylenolide III and Atractylone had been reported to improve inflammation by suppressing the release of pro-inflammatory cytokines and nitric oxide[37, 38]. Combined with our results, AL might improve the COVID-19 by regulating IL-6, IL-1β, MAPK1, MAPK8 and TNF pathway to reduce the "cytokine storm" and help patients recover.
There had been controversy for the therapeutic effects of remdesivir, hydroxychloroquine and other antiviral drugs on COVID-19, but the Food and Drug Administration had approved remdesivir for the treatment of adults and children aged 12 years and above, which suggested that effective drugs against COVID-19 were of great significance. Many active compounds in AL had been proved to have strong antiviral effects[8, 10]. The common targets between AL and COVID-19 were mainly enriched in some antiviral pathways, which suggested that AL exerted therapeutic effects on COVID-19 through regulating these antiviral pathways. Through further analysis, we found that the genes of Module 2 were also enriched in antiviral pathways. These genes included CCNA2, CCND3, HDAC2, CREBBP, CDK4, STAT3, CDK2, MAPK1, JAK1, PRKCB and STAT3, which could be considered as necessary genes for AL against COVID-19.
Another important characteristic of COVID-19 was high infectivity. After experiencing SARS, scientists had found the infectious mechanisms of coronavirus. ACE2 was considered as the most important receptor for SARS-CoV to enter the human body[39]. The spike protein of SARS-CoV-2 used the SARS-CoV receptor ACE2 for facilitating viral entry into target cells[40]. NRP1 was a new host factor for SARS-CoV-2 infection, which could bind furin-cleaved substrates and significantly potentiate SARS-CoV-2 infectivity[41]. Our results suggested that the binding energy of AL6 and AL2 to 3CLpro was less than -7 kcal/mol, which indicated that they could form stable binding with 3CLpro and suppress the activity of SARS-CoV-2. In addition, the binding energy of AL6 and AL38 to ACE2-spike protein complex was less than -7 kcal/mol, which indicated that they could form stable binding with ACE2 to suppress SARS-CoV-2 entry into target cells. Finally, the binding energy of most compounds to NRP1 was less than -6 kcal/mol, meaning that these compounds could bind to NRP1. In short, our results showed that AL2, AL6 and AL38 were active compounds in AL involved in improving the effect of antiviral and decreasing the risk of infection.
However, the present study still remains some limitations. We analyzed the potential mechanisms of AL against COVID-19 based on network pharmacology and molecular docking. But these potential mechanisms need to be verified through rigorous biological experiments. In future research, the experiments in vivo and in vitro will proceed to prove the mechanisms of AL against COVID-19.
4 Conclusion
The COVID-19 pandemic caused by SARS-CoV-2 was still going on. In China, AL-related prescription had been used clinically for against COVID-19. We had explored the potential mechanisms of AL against COVID-19 based on network pharmacology and molecular docking. These mechanisms included the following: AL may treat COVID-19 by improving the "cytokine storm", regulating antiviral pathways and related proteins, and inhibiting the key protein through which the SARS-CoV-2 invades the host cell. Except ACE2, NRP1 was the latest discovered key protein for SARS-CoV-2 infection. We found that compounds of AL could bind to the active site of NRP1, which may reduce the infectivity of SARS-CoV-2. Our research provided a scientific basis for AL and active compounds as potential treatment drugs for COVID-19. We also provided a research direction for the future treatment of SARS-CoV-2 through NRP1.
Conflicts of Interest
The authors declare that they have no known competing financial interests in this paper.
References
- Baloch S, Baloch M A, Zheng T L, et al. The coronavirus disease 2019 (COVID-19) pandemic [J]. Tohoku J Exp Med, 2020, 250(4): 271-278. [CrossRef] [PubMed] [Google Scholar]
- Wang J H, Jing R Z, Lai X Z, et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China [J]. Vaccines, 2020, 8(3): 482. [CrossRef] [Google Scholar]
- Kaptein S J F, Jacobs S, Langendries L, et al. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2–infected hamsters, whereas hydroxychloroquine lacks activity[J]. PNAS, 2020, 117(43): 26955-26965. [CrossRef] [PubMed] [Google Scholar]
- Benton D J, Wrobel A G, Xu P Q, et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion[J]. Nature, 2020, 588(7837): 327-330. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Clausen T M, Sandoval D R, Spliid C B, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2[J]. Cell, 2020, 183(4): 1043-1057. [CrossRef] [PubMed] [Google Scholar]
- Daly J L, Simonetti B, Klein K, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection [J]. Science, 2020, 370(6518): 861-865. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Koonrungsesomboon N, Na-Bangchang K, Karbwang J. Therapeutic potential and pharmacological activities of Atractylodes lancea (Thunb.) DC [J]. Asian Pacific Journal of Tropical Medicine, 2014, 7(6): 421-428. [CrossRef] [PubMed] [Google Scholar]
- Cheng Y, Mai J Y, Hou T L, et al. Antiviral activities of atractylon from atractylodis rhizoma [J]. Molecular Medicine Reports, 2016, 14(4): 3704-3710. [CrossRef] [PubMed] [Google Scholar]
- Wu C R, Jiang X, He S T, et al. Effects of QWBZP on T-cell subsets and their cytokines in intestinal mucosa of HRV infection suckling mice [J]. Journal of Ethnopharmacology, 2010, 131(1): 130-134. [CrossRef] [PubMed] [Google Scholar]
- Gu S H, Li L, Huang H, et al. Antitumor, antiviral, and anti-inflammatory efficacy of essential oils from Atractylodes macrocephala koidz. produced with different processing methods[J]. Molecules (Basel, Switzerland), 2019, 24(16): 2956. [Google Scholar]
- Zheng S C, Baak J P, Li S, et al. Network pharmacology analysis of the therapeutic mechanisms of the traditional Chinese herbal formula Lian Hua Qing Wen in corona virus disease 2019 (COVID-19), gives fundamental support to the clinical use of LHQW [J]. Phytomedicine, 2020, 79: 153336. [CrossRef] [PubMed] [Google Scholar]
- Hopkins A L. Network pharmacology: The next paradigm in drug discovery [J]. Nature Chemical Biology, 2008, 4(11): 682-690. [CrossRef] [PubMed] [Google Scholar]
- Li H, Zhang L. Study on the molecular mechanism of Atractylodes lancea in treating corona virus disease 2019(COVID-19) based on network pharmacology [J]. Journal of Chinese Medicinal Materials, 2020, 43(10): 2608-2612 (Ch). [Google Scholar]
- Ru J L, Li P, Wang J N, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines [J]. Journal of Cheminformatics, 2014, 6: 13. [CrossRef] [PubMed] [Google Scholar]
- Xue R C, Fang Z, Zhang M X, et al. TCMID: Traditional Chinese medicine integrative database for herb molecular mechanism analysis [J]. Nucleic Acids Research, 2012, 41(D1): D1089-D1095. [CrossRef] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules [J]. Scientific Reports, 2017, 7: 42717. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Wang X, Shen Y H, Wang S W, et al. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database [J]. Nucleic Acids Research, 2017, 45(W1): W356-W360. [CrossRef] [PubMed] [Google Scholar]
- Liu X F, Ouyang S S, Yu B, et al. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach [J]. Nucleic Acids Research, 2010, 38(suppl_2): W609-W614. [CrossRef] [PubMed] [Google Scholar]
- Gfeller D, Michielin O, Zoete V. Shaping the interaction landscape of bioactive molecules [J]. Bioinformatics, 2013, 29(23): 3073-3079. [CrossRef] [PubMed] [Google Scholar]
- Daina A, Michielin O, Zoete V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules [J]. Nucleic Acids Research, 2019, 47(W1): W357-W364. [CrossRef] [PubMed] [Google Scholar]
- Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: From gene data mining to disease genome sequence analyses[J]. Current Protocols in Bioinformatics, 2016, 54: 1.30.1-1.30.33. [Google Scholar]
- Hamosh A, Scott A F, Amberger J S, et al. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders [J]. Nucleic Acids Research, 2005, 33(suppl_1): D514-D517. [Google Scholar]
- Szklarczyk D, Gable A L, Lyon D, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets [J]. Nucleic Acids Research, 2018, 47(D1): D607-D613. [Google Scholar]
- Chin C H, Chen S H, Wu H H, et al. cytoHubba: Identifying hub objects and sub-networks from complex interactome [J]. BMC Systems Biology, 2014, 8(4): S11. [CrossRef] [PubMed] [Google Scholar]
- Bader G D, Hogue C W V. An automated method for finding molecular complexes in large protein interaction networks[J]. BMC Bioinformatics, 2003, 4: 2. [CrossRef] [PubMed] [Google Scholar]
- Huang D W, Sherman B T, Lempicki R A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists [J]. Nucleic Acids Research, 2008, 37(1): 1-13. [Google Scholar]
- Li J L, Luo H, Liu X K, et al. Dissecting the mechanism of Yuzhi Zhixue Granule on ovulatory dysfunctional uterine bleeding by network pharmacology and molecular docking[J]. Chinese Medicine, 2020, 15: 113. [CrossRef] [PubMed] [Google Scholar]
- Hu S L, Wang J, Zhang Y J, et al. Three salvianolic acids inhibit 2019-nCoV spike pseudovirus viropexis by binding to both its RBD and receptor ACE2 [J]. Journal of Medical Virology, 2021, 93(5): 3143-3151. [CrossRef] [PubMed] [Google Scholar]
- Bardou P, Mariette J, Escudié F, et al. Jvenn: An interactive Venn diagram viewer [J]. BMC Bioinformatics, 2014, 15(1): 293. [Google Scholar]
- Yang Y, Islam M S, Wang J, et al. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective [J]. International Journal of Biological Sciences, 2020, 16(10): 1708-1717. [Google Scholar]
- Zhang Y, Xu Q H, Sun Z Y, et al. Current targeted therapeutics against COVID-19: Based on first-line experience in China [J]. Pharmacological Research, 2020, 157: 104854. [CrossRef] [PubMed] [Google Scholar]
- Luo C H, Ma L L, Liu H M, et al. Research progress on main symptoms of novel coronavirus pneumonia improved by traditional Chinese medicine [J]. Frontiers in Pharmacology, 2020, 11: 556885. [Google Scholar]
- Prajitha N, Athira S, Mohanan P. Pyrogens, a polypeptide produces fever by metabolic changes in hypothalamus: Mechanisms and detections [J]. Immunology Letters, 2018, 204: 38-46. [CrossRef] [PubMed] [Google Scholar]
- Reyes-Gibby C C, Wang J, Spitz M, et al. Genetic variations in interleukin-8 and interleukin-10 are associated with pain, depressed mood, and fatigue in lung cancer patients [J]. Journal of Pain and Symptom Management, 2013, 46(2): 161-172. [CrossRef] [PubMed] [Google Scholar]
- Kim J S, Lee J Y, Yang J W, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19 [J]. Theranostics, 2021, 11(1): 316-329. [Google Scholar]
- Park E J, Park S W, Kim H J, et al. Dehydrocostuslactone inhibits LPS-induced inflammation by p38MAPK-dependent induction of hemeoxygenase-1 in vitro and improves survival of mice in CLP-induced sepsis in vivo [J]. International Immunopharmacology, 2014, 22(2): 332-340. [CrossRef] [PubMed] [Google Scholar]
- Wang C H, Duan H J, He L C. Inhibitory effect of atractylenolide I on angiogenesis in chronic inflammation in vivo and in vitro [J]. European Journal of Pharmacology, 2009, 612(1/2/3): 143-152. [CrossRef] [PubMed] [Google Scholar]
- Li C Q, He L C, Jin J Q. Atractylenolide I and atractylenolide III inhibit Lipopolysaccharide-induced TNF-alpha and NO production in macrophages [J]. Phytotherapy Research PTR, 2007, 21(4): 347-353. [CrossRef] [PubMed] [Google Scholar]
- Li W H, Moore M J, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus [J]. Nature, 2003, 426(6965): 450-454. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [J]. Cell, 2020, 181(2): 271-280. [CrossRef] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Ojha R, Pedro L D, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity [J]. Science, 2020, 370(6518): 856-860. [CrossRef] [PubMed] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Identification of the common genes and construction of PPI network (a) Identification of the AL-target and disease-related genes by taking an intersection of AL target genes and COVID-19-related genes; (b) Construction of PPI network of common genes by STRING database |
| In the text | |
 |
Fig. 2 The MCODE analysis of PPI network of common genes (a) The genes of module 1 with the score of 16.211; (c) The genes of module 2 with the score of 9.684; (e) The genes of module 3 with the score of 5.333; (b), (d), (f):The KEGG pathway analysis of module 1-3 |
| In the text | |
 |
Fig. 3 The visualization of GO and KEGG pathway analysis (a) GO enrichment analysis; (b) KEGG pathway analysis; BP: biological process; MF: molecular function; CC: cellular component |
| In the text | |
 |
Fig. 4 Compound-Target-Pathway network diagram (a) and hub genes (b) In (a), red rounds represent the 84 common targets of AL against COVID-19, blue squares triangles represent the 39 active compounds of AL, and green triangles represent the 10 key pathways of AL against COVID-19. In (b), lines represent protein-protein interactions present, and the color of rounds from red to yellow represent the score values varying from higher to lower |
| In the text | |
 |
Fig. 5 Molecular docking mode of 3 key targets and compounds with lowest binding energy (a) AL6-3CLpro; (b) AL6-NRP1; (c) AL38-ACE2-spike protein complex. Hydrogen bonding was shown by green dashed line |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


