| Issue |
Wuhan Univ. J. Nat. Sci.
Volume 30, Number 1, February 2025
|
|
|---|---|---|
| Page(s) | 91 - 102 | |
| DOI | https://doi.org/10.1051/wujns/2025301091 | |
| Published online | 12 March 2025 | |
Materials Science
CLC number: TB333
Preparation and Characterization of Magnetic Ag3PO4/CoFe1.95Dy0.05O4 S-Scheme Heterojunction
磁性Ag3PO4/CoFe1.95Dy0.05O4 S型异质结的制备与表征
School of Chemistry and Materials Engineering, Huainan Normal University, Huainan 232038, Anhui, China
† Corresponding author. E-mail: 13956468746@163.com; 573543828@qq.com
Received:
1
May
2024
The development of efficient photocatalysts is of paramount importance for the photocatalytic degradation of organic compounds. An effective approach is to construct heterojunctions with tight interface structures in order to enhance interfacial charge transfer and achieve high photocatalytic activity. A magnetically recyclable photocatalytic composite, comprising Ag3PO4/CoFe1.95Dy0.05O4 (AP/CFDO) S-scheme heterojunction, was synthesized using a simple hydrothermal method. The composition, microstructure and photoelectrochemical properties of the nanocomposites were comprehensively characterized by various advanced characterization methods. The photocatalytic activity of the AP/CFDO nanocomposites was investigated by subjecting methylene blue (MB) to degradation. The results demonstrated that AP/CFDO exhibited high degradation efficiency in the catalytic degradation of MB, with a degradation efficiency of 99.8% achieved within 30 min under visible light irradiation. Furthermore, after five repeated experiments, the degradation efficiency of MB under visible light irradiation remained at 90%. Furthermore, the degradation process followed the first-order kinetic reaction model, with a rate constant of 0.140 42 min-1, which was 2.47 and 10.77 times that of Ag3PO4 (AP, 0.056 78 min-1) and CFDO (0.013 04 min-1). This phenomenon can be attributed to the S-scheme heterojunction constructed between AP and CFDO, which enables the effective spatial separation and transfer of photogenerated carriers. Finally, the reaction mechanism of photocatalytic degradation of MB was studied by adding different free radical scavengers. The results of capture experiments showed that superoxide radicals and hydroxyl radicals were the main active substances in the process of photocatalytic degradation.
摘要
开发高效的光催化剂对有机原料的光催化降解至关重要。一种有效的方法是构建具有紧密界面结构的异质结,以增强界面电荷转移,实现较高的光催化活性。采用简单的水热法合成了一种磁性Ag3PO4/CoFe1.95Dy0.05O4(AP/CFDO)S型异质结光催化复合材料,对其组成、微观结构和光电化学性质进行了表征。通过亚甲基蓝(MB)降解,研究了AP/CFDO的光催化活性。结果表明,AP/CFDO在MB的催化降解过程中具有较高的降解效率,在可见光照射下30 min范围内降解效率达到99.8%。此外,经过5次重复实验,MB在可见光照射下的降解效率仍保持在90%。降解过程遵循一级动力学反应模型,速率常数为0.140 42 min-1,分别为AP(0.056 78 min-1)和CFDO(0.013 04 min-1)的2.47倍和10.77倍,这可以归因于AP和CFDO之间构建的S型异质结,使光生载流子能够实现有效的空间分离和转移。最后,通过添加不同的自由基清除剂,研究了光催化降解MB的反应机理。捕获实验结果表明,超氧自由基(·O )和羟基自由基(·OH)是光催化降解过程中的主要活性物质。
)和羟基自由基(·OH)是光催化降解过程中的主要活性物质。
Key words: Ag3PO4/CoFe1.95Dy0.05O4 / photocatalyst / cyclic degradation / magnetic separation recovery
关键字 : Ag3PO4/CoFe1.95Dy0.05O4 / 光催化剂 / 循环降解 / 磁分离回收
Cite this article: LIU Qingwang, XU Mai, MENG Ying. Preparation and Characterization of Magnetic Ag3PO4/CoFe1.95Dy0.05O4 S-Scheme Heterojunction[J]. Wuhan Univ J of Nat Sci, 2025, 30(1): 91-102.
Biography: LIU Qingwang, male, Master, Senior experimentalist, research direction: preparation and application of photocatalytic composite materials. E-mail: qwliu2006@163.com
Foundation item: Supported by 2023 Anhui Modern Coal Processing Technology Research Institute Open Fund Project (MTY202305)
© Wuhan University 2025
 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
0 Introduction
In recent years, the rapid development of industrialization has resulted in a significant increase in the discharge of organic wastewater into the environment. This has led to serious damage to the ecological environment and human health[1-3]. Consequently, there is a pressing need to identify an effective and safe method to remove these organic pollutants. Semiconductor photocatalytic degradation technology is emerging as a promising solution to the problem of environmental pollution[4,5].
The field of photocatalytic solar energy conversion has recently garnered significant interest due to its cost-effectiveness, minimal energy consumption, and potential for environmental protection. However, there are several limitations and shortcomings associated with semiconductor photocatalytic technology, including a wide band gap energy, a narrow visible light response range, and a lack of catalyst reusability. These challenges must be addressed for the technology to reach its full potential[6-8]. For instance, titanium dioxide has been identified as an effective photocatalyst for water disinfection and the degradation of organic pollutants. However, due to its large band gap, it necessitates ultraviolet irradiation, which constrains its practical application in photocatalytic applications[9-11]. While semiconductor photocatalytic technology exhibits certain limitations, and photocatalytic technology remains a pivotal environmental protection technology, exhibiting certain effects and application prospects in practical applications. Furthermore, in the context of green chemistry, the recyclability and stability of photocatalysts must also be considered. In conclusion, it is highly desirable to develop new photocatalysts with a broad visible light response range, high reactive oxygen species (ROS) generation capacity, simple recovery, and excellent stability.
Ag3PO4, a novel semiconductor with high visible light absorption in the solar spectrum, has garnered significant interest in the field of photocatalysis[12]. Ag3PO4 exhibits remarkable photocatalytic ability to degrade organic dyes under visible light irradiation. However, the photocatalytic activity of pure Ag3PO4 may be constrained by its relatively low photogenerated charge separation/transport efficiency and limited light capture ability[13,14]. The combination of a target semiconductor with an appropriate semiconductor to form a specific heterostructure composite material allows for the effective adjustment of the energy band structure and surface charge distribution, thereby improving the overall photoelectrocatalytic performance. Furthermore, the recycling problem of composite materials can be solved by coupling high ferromagnetic semiconductors to prepare high magnetic composite materials[15,16]. Consequently, photocatalytic materials with high degradation efficiency and high stability of magnetic recycling cycle degradation are in accordance with the environmental protection concept of sustainable development.
Magnetic semiconductor photocatalysts, such as spinel ferrite ( MFe2O4, M=Co, Cu, Zn, Ni, Mn ), have been widely studied in the field of photocatalysis due to their high ferromagnetism and excellent chemical stability. It is found that the doping of rare earth metal elements such as (Sm, Dy, Y, Ce) improves the magnetization saturation visible light adsorption effect of spinel ferrite CoFe2O4. Therefore, the doping of rare earth metal elements in spinel CoFe2O4 is one of the effective ways to improve the photocatalytic degradation activity. The improvement of photocatalytic performance of spinel CoFe2O4 is because the high magnetic moment of rare earth metal cations leads to the rearrangement of catalyst sites, which increases the light absorption and carrier density of the catalyst[17]. The catalytic activity of CoFe2O4 can be improved by using dysprosium (Dy) to partially replace Fe in CoFe2O4 to prepare CFDO[18]. At the same time, CFDO nanoparticles have significantly improved magnetic properties. Under the action of an external magnetic field, they can be easily recovered from the suspension and can be reused for many times. Therefore, MFe2O4 doped with rare earth elements can improve its saturation magnetization and broaden its visible light response range, achieve rapid electron and hole electron and hole separation and slow charge recombination, thereby enhancing photocatalytic performance[19,20]. The synthesis of photocatalysts with high efficiency, magnetic recovery and cyclic degradation stability by coupling AP with CFDO as the substrate has industrial application prospects.
In this paper, we synthesized a novel type of AP/CFDO S-scheme heterojunction composite photocatalyst via the hydrothermal method, and analyzed the crystal properties, microscopic morphology, and photoelectrochemical properties of AP/CFDO using a series of characterization techniques. Furthermore, the photocatalytic performance and cyclic degradation stability of the composite catalyst were evaluated by subjecting methylene blue (MB) to visible light irradiation. Finally, the S-type electron transfer mechanism of AP/CFDO composites was proposed and proved by matching band structure, active species capture experiment and work function analysis.
1 Experimental
1.1 Reagents
Cobalt nitrate hexahydrate (Co(NO3)2·6H2O), ferric nitrate nonahydrate (Fe(NO3)3·9H2O), citric acid (C6H8O7), dysprosium nitrate hexahydrate (Dy(NO3)3·6H2O), silver nitrate (AgNO3), polyvinylpyrrolidone (PVP), disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O) and MB were purchased from China Pharmaceutical Group. The reagents used were analytically pure, and the solution was prepared with ultrapure water.
1.2 Synthesis of Samples
1.2.1 Preparation of CFDO
Weigh 2.85 g cobalt nitrate hexahydrate, 7.43 g ferric nitrate monohydrate, 6.25 g citric acid and 0.018 g dysprosium nitrate hexahydrate in 150 mL beaker, then add 50 mL ultrapure water, constant temperature magnetic stirring reaction for 1 h. Then ammonia was added dropwise to the beaker to adjust the pH to 6-7. The reaction was carried out at a constant temperature of 100 ℃ for 24 h to obtain a xerogel, which was then placed in a crucible and heated to 700 ℃ at a rate of 2.5 ℃/min in a muffle furnace for 3 h. The sample was then cooled to room temperature. The target sample was obtained by grinding.
1.2.2 Preparation of AP/CFDO
0.2 g CFDO was weighed and placed in a 150 mL beaker, and then 100 mL deionized water was added to the beaker. After the beaker was placed in an ultrasonic cleaner for 10 min, silver nitrate (0.840 g), polyvinylpyrrolidone (1.20 g) and disodium hydrogen phosphate dodecahydrate (1.790 7 g) were added to the beaker. Then it was ultrasonically mixed for 1 h. The reactants were moved into a 150 mL high-pressure reactor, and then the reactor was placed in a drying oven at 200 ℃ for 24 h, and then cooled to room temperature. Then, the reaction product was filtered to obtain the filter, and the filter was washed with water and ethanol three times respectively. Then the filter was placed in a vacuum drying oven at 60 ℃ for 10 h, and the target sample was obtained by grinding. Ag3PO4 samples were synthesized by the same method without adding CFDO.
1.3 Analytic Characterization
X-ray diffractometer (XRD, Rigaku Ultima IV, Japan Co., Ltd.) was used to examine the physical purity and crystal structure of the samples obtained. A scanning electron microscope (FE-SEM , NANOSEM 450, FEI company) was used for the morphological study of the samples. The samples were characterized by TEM using a high definition electron microscope (HRTEM, H-7000FA, Hitachi, Japan). A UV-visible spectrophotometer (UV-Vis DRS, UV-2550, Shimadzu, Japan) was used to characterize the energy band structure. Photoluminescence spectra were measured using an in fluorescence spectrophotometer (PL, Jasco FP-6500, Japan). X-ray photoelectron spectroscopy (XPS, Axis Ultra) was used to collect data on the chemical composition and elemental valence states of the sample surface. The electrochemical impedance (EIS) of the sample was measured by an electrochemical workstation (CHI660B). Magnetic measurements were performed at room temperature using a vibrating sample magnetometer (VSM, Magnetic Daneshpajoh Kashan Co., Iran) with a maximum magnetic field of 20 kGs. The photocatalytic performance was tested using a photocatalytic equipment (ZQ-GHX-V, Qiangqiao, Shanghai).
1.4 Evaluation of Photocatalytic Activity and Stability
A total of 50 mg of each catalyst was added to 50 mL of MB solution containing 1.0×10-5 mol/L, respectively, and stirred for 60 min in the absence of light in order to achieve equilibrium between the photocatalyst and the dye. Subsequently, the dye solution was subjected to irradiation with a 500 W xenon lamp for the purpose of evaluating its catalytic degradation potential. During the photocatalytic process, 5.0 mL of the MB solution was withdrawn at 5-min intervals and centrifuged for 3 min. The supernatant was then collected and filtered, and the absorbance of the supernatant was measured at the maximum absorption wavelength of MB (550 nm) by UV-vis spectrometer. Furthermore, the cyclic degradation stability of the photocatalysts was investigated through the implementation of cyclic photocatalytic experiments. Under the same experimental conditions as the catalytic degradation experiments, five consecutive catalytic degradation experiments were performed to examine the changes in photocatalytic performance.
2 Results and Discussion
2.1 Structure and Morphology Analysis
Figure 1(a) illustrates the XRD patterns of the prepared AP, CFDO and AP/CFDO samples. In the XRD mode, a series of diffraction peaks 2θ=18.29°, 30.08°, 35.43°, 56.94°, and 62.53° of the prepared CFDO samples can be indexed to the (003), (104), (113), (125), and (208) crystal planes (Fig.1(b)), which are consistent with the standard diffraction data of CoFe2O4 (PDF#79-1744), suggesting that dysprosium doping does not change the CoFe2O4 crystal shape. A series of diffraction peaks 2θ=20.88°, 29.77°, 33.29°, 36.59°, 47.79°, 52.70°, 55.02°, 57.28°, and 61.64° of the prepared AP samples can be indexed to (110), (220), (210), (211), (310), (222), (320), (321), and (400) crystal planes, in agreement with the standard diffraction data for AP (PDF#06-0505), which indicates successful preparation of AP. From the diffractogram of AP/CFDO binary composite, it can be observed that the diffraction peaks are reduced in intensity from the pure AP diffraction peaks, which matches the (113) crystal plane of CFDO at 2θ=35.43°. The other diffraction peaks of CFDO do not appear in the complexes, which may be due to the low content or low crystallinity of CFDO in the complexes[21,22]. In addition, the peak position of AP is not shifted in the diffraction pattern of AP/CFDO composites. It indicates that the introduction of CFDO has little effect on the crystal structure of silver phosphate. Therefore, it can be demonstrated that AP/CFDO composite photocatalysts have been successfully prepared.
 |
Fig. 1 XRD patterns of different samples (a) and CFDO after magnification (b) |
2.2 Morphological Analysis of the Samples
Figure 2(a), (b), and (c) show SEM photos of pure AP, CFDO nanomaterials, and AP/CFDO nanocomposites. From Fig. 2(a), it can be seen that the pure AP is in the shape of a polygonal polygon with an average diameter of about 0.5 μm. From Fig. 2(b), it can be seen that the CFDO is in the shape of spherical nanoparticles, with an average size of about 100 nm. from Fig. 2(c), it can be seen that the CFDO is tightly embedded in AP particles, and the two are in close contact with each other, which means that they have been compounded into a nanoscale composite material. As seen in Fig. 2(d), the (113) crystal plane spacing of CFDO is 0.219 6 nm, indicating that the AP/CFDO forms a heterojunction.
 |
Fig. 2 Morphological analysis of the samples (a), (b), (c) SEM images of AP, CFDO and AP/CFDO; (d) HRTEM image of the AP/CFDO; (e) EDS mapping of the AP/CFDO |
In addition, EDS elemental imaging (Fig. 2(e)) shows that the composite is composed of Co, Fe, Dy, O, Ag and P elements. Overall, the distribution of the elements is relatively uniform, indicating that the composites have been successfully prepared.
2.3 XPS Characterization
The elemental composition and chemical state of the magnetic AP/CFDO composite photocatalyst samples were studied by XPS technology. The test results are shown in Fig. 3. From the spectra of elemental Co in Fig.3(a), it can be analyzed that the binding energy has two peaks at 780.80 and 796.96 eV, which can be corresponded to the Co 2p3/2 and Co 2p1/2 peaks, respectively, which proves that elemental Co has the main valence state of Co2+ in the complex. From the spectrum of elemental Fe (Fig.3(b)), it can be seen that the binding energy has two peaks 711.70 and 725.10 eV which can be corresponded to the peaks of Fe 2p1/2 and Fe 2p3/2, respectively. It is proved that the main valence state of Fe element in the complex is Fe3+. The spectral peaks located at 1 296.89 and 1 334.78 eV in Fig.3(c) are attributed to the Dy 3d5/2 characteristic peaks[23]. In the spectrogram of elemental Ag (Fig.3(d)), the binding energy has two peaks 367.90 eV and 373.80 eV can be corresponded to Ag3d5/2 and Ag3d3/2 peaks, respectively, which proves that the main valence state of elemental Ag in the AP/CFDO complex is Ag+. The spectrum of element P is shown in Fig.3(e), and the binding energy of 132.90 eV can be corresponded to the P 2p peak, which is consistent with the P element from PO . From the spectrum of O element in Fig.3(f), the peaks at 530.60 and 532.50 eV should be attributed to the oxygen in the AP lattice and the surface oxygen species of the composites, including hydroxyl or carboxyl oxygen groups[24,25]. The above indicates that the photocatalytic composites have been successfully prepared.
. From the spectrum of O element in Fig.3(f), the peaks at 530.60 and 532.50 eV should be attributed to the oxygen in the AP lattice and the surface oxygen species of the composites, including hydroxyl or carboxyl oxygen groups[24,25]. The above indicates that the photocatalytic composites have been successfully prepared.
 |
Fig. 3 XPS spectra of the as-prepared AP/CFDO composite sample |
2.4 Energy Band Structure of the Samples
The light absorption properties of CFDO, AP and AP/CFDO composites were investigated by UV-vis DRS spectroscopy (Fig.4(a)). CFDO exhibits excellent absorption throughout the visible region. Pure AP shows an absorption edge at about 530 nm. After the composite of CFDO and AP, the absorption efficiencies in both the UV region and the visible region were improved, and the increase in the intensity of the visible absorption and the UV absorption were obvious because CFDO was decorated on the surface of the AP particles. It indicates that AP/CFDO should have higher photocatalytic activity for the degradation of pollutants. Based on the plot of (αhν)2 versus (hν) (Fig.4(b)), the AP and CFDO forbidden bandwidths can be calculated to be 2.51 and 1.78 eV, respectively. Then, according to the VB-XPS spectra (Fig.4(c),(d)), the EVB-XPS of AP and CFDO are 3.02 and 1.16 eV, respectively. Therefore, using the formula EVB-NHE =φ+EVB-XPS-4.44 (EVB-NHE is EVBvs. the normal hydrogen electrode (NHE) potential, φ is the electron work function of the XPS analyzer with a value of 4.55, and EVB-XPS is the VB value tested by VB-XPS), it can be obtained that the EVB of AP and CFDO are 3.13 and 1.27 eV, respectively. Finally, the ECB of AP and CFDO were calculated to be 0.62 and -0.51 eV for AP and CFDO, respectively, based on the equation: EVB-ECB=Eg[26,27].
 |
Fig. 4 Light absorption properties and energy band structure of CFDO, AP and AP/CFDO composites (a) UV-vis diffuse reflectance absorption spectra of the samples; (b) Plots of (αhν)2vs. hν of samples; (c) and (d) VB-XPS curves of AP and CFDO |
2.5 Photoelectric Properties of the Sample
The photoluminescence (PL) signal originates from the compounding process of photogenerated electron-hole pairs, and the high fluorescence intensity indicates that more photogenerated electron-hole pairs are compounded[28,29]. Figure 5(a) shows the PL spectra of different samples, the fluorescence signal intensity of AP is the largest, followed by CFDO, suggesting that the photogenerated electron-hole pairs of a single semiconductor are heavily compounded. However, the fluorescence signal intensity of the sample AP/CFDO was significantly reduced to a minimum after composite. This indicates that the photogenerated electron-hole pair composite is significantly alleviated. CFDO can inhibit the photogenerated electron-hole pair complexation in AP, thus improving the photocatalytic performance of the material. Figure 5(b) shows the electrochemical impedance spectroscopy (EIS) test results of different samples, which shows that the impedance arc of AP is the largest, followed by AP/CFDO, and sample CFDO is the smallest. It is indicated that the composite of CFDO and AP can reduce the charge transfer resistance of AP, and the migration of interfacial carriers in the composite samples is more convenient, which is beneficial to the photocatalytic activity[30,31]. The qualitative analysis of the semiconductor type and flat-charged potentials of the samples is conducted using the Mott-Schottky (M-S) curves. The M-S curves of AP and CFDO are presented in Fig.5(c) and (d), respectively. The slopes of the two curves are positive, indicating that both are n-type semiconductors, and the intercepts of the tangent lines of the straight line portion of the M-S curves on the x-axis are 0.42 and -0.71 eV, respectively. Therefore, according to the formula ENHE=EAg/AgCl+0.197, the conduction band positions of AP and CFDO are approximated to be 0.62 and -0.51 eV[32,33], respectively.
 |
Fig. 5 Fluorescence spectra (a) and EIS profile (b) of samples, and Mott-Schottky curves of AP (c) and CFDO (d) |
2.6 Photocatalytic Activity and Degradation Stability
2.6.1 Photocatalytic activity
The photocatalytic performance of CFDO, AP and AP/CFDO samples was evaluated by degrading MB dye wastewater using a xenon lamp (500 W) to simulate a solar light source. Firstly, photocatalyst (50 mg) was added to the beaker, and after stirring for 30 min to reach the adsorption equilibrium, the photocatalytic reaction was carried out under the condition of xenon lamp irradiation. The degradation results are shown in Fig. 6(a) after AP doping with CFDO, the degradation of MB dyes by AP/CFDO complexes were all better than that of pure AP and CFDO, indicating that the doping of these two substances helped to improve AP activity and enhance the ability of the composites to degrade MB dyes. Then, the fitted curve of the primary reaction kinetics was obtained by linear fitting transformation (Fig. 6(b)), where k is the slope of the fitted curve, indicating the apparent rate constant of photodegradation of MB by the photocatalyst. After 30 min of illumination, the k of photodegradation MB of AP/CFDO reached 0.140 42 min-1, which was 2.47 and 10.77 times higher than that of pure AP (k=0.056 78 min-1) and CFDO (k=0.013 04 min-1), respectively, indicating that the photocatalytic reaction rate of the composite sample was significantly enhanced.Figure 6(c) shows the effect of pH on the degradation efficiency of MB in the presence of AP/CFDO. From Fig. 6(c), it can be seen that the degradation efficiency is lowest under neutral conditions, which is mainly due to the different effects of pH on the microstructure, surface charge, edge position and adsorption of organic molecules by AP/CFDO. When the pH value is less than or more than 6, the surface of the dye molecules is positively or negatively charged, and the AP/CFDO composite catalyst can promote the adsorption between the dye molecules and the catalyst, thus accelerating the degradation reaction. Therefore, the catalytic performance of AP/CFDO under acidic and alkaline conditions is better than that under neutral conditions, which is the result of the different reasons mentioned above. This also implies that the prepared catalysts have wide pH adaptability.
 |
Fig. 6 Visible photocatalytic degradation of MB (a), first-order kinetic curves for different samples (b), and effect of pH (c) and different anions (d) on the degradation efficiency of AP/CFDO |
The presence of a large number of coexisting inorganic ions in polluted water may affect the catalytic effect. Therefore, Cl , HCO
, HCO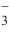 and NO
and NO were selected as representative substances to investigate their effects on the degradation of MB. As shown in Fig. 6(d), all the above three substances inhibited MB degradation to some extent. In particular, the presence of HCO
were selected as representative substances to investigate their effects on the degradation of MB. As shown in Fig. 6(d), all the above three substances inhibited MB degradation to some extent. In particular, the presence of HCO greatly inhibited MB degradation, and the removal rate of MB decreased from 99.5% to 60.2% in less than 30 min. The inhibitory effect of HCO
greatly inhibited MB degradation, and the removal rate of MB decreased from 99.5% to 60.2% in less than 30 min. The inhibitory effect of HCO on MB degradation may be due to its interaction with ·OH to form ·CO3 with lower oxidizing capacity. In addition, the inhibitory effect of Cl
on MB degradation may be due to its interaction with ·OH to form ·CO3 with lower oxidizing capacity. In addition, the inhibitory effect of Cl on MB degradation may be due to its interaction with ·OH to form ·Cl. NO
on MB degradation may be due to its interaction with ·OH to form ·Cl. NO can also inhibit the degradation reaction, and 83.2% of the MB can still be removed within 30 min, indicating that the effect of NO
can also inhibit the degradation reaction, and 83.2% of the MB can still be removed within 30 min, indicating that the effect of NO on the reaction is relatively weak. Therefore, the anion is an important factor in the catalytic degradation system.
on the reaction is relatively weak. Therefore, the anion is an important factor in the catalytic degradation system.
2.6.2 Stability of cyclic degradation
Figure 7(a) shows the hysteresis of the CFDO and AP/CFDO samples at room temperature. It is clear from Fig. 7(a) that the hysteresis loops of the samples are characteristic of the ferromagnetic behaviour and that the magnetic saturation strength (Ms) of AP/CFDO is lower than that of pure CFDO. In general, the Ms of a sample depends mainly on the content of the magnetic component. AP/CFDO contains less magnetic component than pure CFDO, so its Ms value is relatively low[34]. However, the magnetic AP/CFDO composite catalyst can still be separated from the nonhomo-geneous reaction system by the addition of a magnet as shown in the inset in Fig.7(a).
 |
Fig. 7 Cyclic degradation stability of the samples (a) Magnetisation curves of the samples (insets are photographs of the solutions before and after magnetic field separation); (b) Cyclic performance curves of the samples for MB degradation; (c) Comparative XRD spectra of AP/CFDO before and after the cyclic degradation experiments |
The degradation of MB dyes was cycled using AP/CFDO composite samples under the same conditions as the photocatalytic degradation experiments. After the completion of a single degradation reaction, firstly, the recovery of the sample is accomplished by using magnets, and then again by centrifugation and filtration to ensure that the minimum amount of sample is lost. Finally, five photocatalytic reactions were carried out under the same conditions to investigate the cyclic degradation performance of the prepared samples. Figure 7(b) shows the stability of the magnetic composite photocatalyst in catalyzing MB degradation. The degradation rate of MB was still as high as 90% after five cycles of reaction of the composite catalyst. It indicates that the magnetic composite photocatalyst has high degradation stability. The introduction of magnetic CFDO makes the composite catalyst magnetic, which facilitates recycling through magnets and reduces catalyst loss during multiple cycles of the reaction.
The recyclability of AP/CFDO heterojunctions was examined by cyclic degradation of MB under 500 W xenon lamp irradiation under the same conditions. After each test, the photocatalysts were recycled, cleaned and dried. As can be seen from Fig.7(c), the XRD patterns before and after five cyclic degradations were almost unchanged, indicating that the photocatalyst has a stable structure in the cyclic degradation system.
2.7 Enhancement Mechanism
2.7.1 Free radical trapping experiments
The free radical trapping experiments are similar to the photocatalytic degradation of organic pollutants, except that disodium ethylenediaminetetraacetic acid (EDTA-2Na, 50 mL, 10 mmol/L), isopropanol (IPA, 50 mL, 10 mmol/L), and p-benzoquinone (BQ, 50 mL, 0.1 mmol/L) are added prior to the degradation as h+, ·OH and ·O traps, respectively. As shown in Fig.8(a), 99.98% of MB is photocatalytically degraded by AP/CFDO after 30 min of light irradiation, and the removal efficiencies of MB are 71.30%, 40.98%, and 28.50% after the addition of EDTA-2Na, IPA, and BQ, respectively, indicating that ·OH and ·O
traps, respectively. As shown in Fig.8(a), 99.98% of MB is photocatalytically degraded by AP/CFDO after 30 min of light irradiation, and the removal efficiencies of MB are 71.30%, 40.98%, and 28.50% after the addition of EDTA-2Na, IPA, and BQ, respectively, indicating that ·OH and ·O are the main active species and h+ plays a secondary role in the CFDO composite system. The presence of ·O
are the main active species and h+ plays a secondary role in the CFDO composite system. The presence of ·O and ·OH active radicals in the MB oxidative degradation system was detected by ESR of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) under visible light irradiation. As illustrated in Fig.8(b), the catalytic degradation reaction system exhibited the presence of ·O
and ·OH active radicals in the MB oxidative degradation system was detected by ESR of 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) under visible light irradiation. As illustrated in Fig.8(b), the catalytic degradation reaction system exhibited the presence of ·O and ·OH radicals, indicating their involvement in the degradation process.
and ·OH radicals, indicating their involvement in the degradation process.
 |
Fig. 8 Photocatalytic radical trapping degradation curves (a), ESR spectra of O and ·OH (b), and VB-XPS curves of AP (c) and CFDO (d) and ·OH (b), and VB-XPS curves of AP (c) and CFDO (d)
|
2.7.2 Work function analysis of the samples
Determination of the inter-semiconductor material work function is important for analyzing the direction of charge transfer at the material interface. Figure 8(c) and (d) show the figure of merit functions of AP and CFDO obtained by small-angle valence band XPS spectroscopy (VB-XPS) measurements, respectively. When two semiconductor materials are in contact, electrons are easily attracted to the material with the larger figure of merit, and conversely, the material with the smaller figure of merit tends to lose electrons until the Fermi energy levels of the two materials reach equilibrium. As a result, the surface of the material with a small figure of merit is positively charged and the surface of the material with a large figure of merit is negatively charged, resulting in a built-in electric field at the contact interface. According to the equation Φ = ΔV+φ (Φ is the work function of the sample, φ is 4.55 eV), ΔV is obtained from the distance between IP1 and IP2 (IP1 is the point of change of the binding energy with respect to the baseline, and IP2 is the midpoint of the corresponding Fermi fringe curve). The distance between the two IP points is calculated to be 1.39 and 1.27 eV for AP and CFDO, respectively. Therefore, the power functions of AP and CFDO are 5.94 and 5.82 eV, respectively[35, 36].
2.7.3 Catalytic degradation mechanism
Based on the above analysis, the mechanism of charge transfer of AP/CFDO photocatalysts was proposed to be an S-scheme heterojunction mechanism (Fig. 9). When AP is in contact with CFDO, the Fermi energy level of CFDO is lower than that of AP. Consequently, electrons on the surface of AP tend to transfer to CFDO, ultimately reaching the dynamically equilibrated Fermi energy level. This results in the formation of a built-in electric field pointing from CFDO to AP at the interface. Additionally, it causes the bending of the energy bands[37-38]. At this time, the photogenerated electrons and holes generated by photoexcitation will be directed by IEF. Driven by the IEF and band bending effect, the photogenerated holes in the valence band of CFDO tend to complex with the photogenerated electrons in the AP conduction band at the interface and annihilate. The strongly oxidizing holes in the VB of AP and the strongly reducing electrons in the CB of CFDO are preserved. Therefore, the AP/CFDO heterojunction not only effectively inhibits the photogenerated carrier complexation, but also preserves the strong redox ability of the carriers. Meanwhile, in the AP/CFDO heterojunction system, the conduction band potential of CFDO (ECB = -0.51 eV) is sufficiently negative, and the photogenerated electrons on CFDO are able to react with dissolved oxygen to form ·O (E(O2/·O2- ) =-0.33 eV (vs. NHE)). In addition, the valence band potential of AP (EVB=3.13 eV) was positive enough to oxidise OH- to ·OH (E(OH-/·OH)=1.99 eV (vs. NHE)). Thus, ·O
(E(O2/·O2- ) =-0.33 eV (vs. NHE)). In addition, the valence band potential of AP (EVB=3.13 eV) was positive enough to oxidise OH- to ·OH (E(OH-/·OH)=1.99 eV (vs. NHE)). Thus, ·O and ·OH are involved in MB degradation, which is consistent with the experimental results of free radical trapping[39-42]. Therefore, based on the comprehensive analysis of the energy band structure, electron transfer, figure of merit and active group detection results of AP/CFDO, the mechanism of charge transfer in AP/CFDO photocatalysts is S-scheme heterojunction mechanism.
and ·OH are involved in MB degradation, which is consistent with the experimental results of free radical trapping[39-42]. Therefore, based on the comprehensive analysis of the energy band structure, electron transfer, figure of merit and active group detection results of AP/CFDO, the mechanism of charge transfer in AP/CFDO photocatalysts is S-scheme heterojunction mechanism.
 |
Fig. 9 Mechanism of AP/CFDO photocatalytic degradation of MB |
3 Conclusion
Magnetic AP/CFDO S-scheme heterojunction composite photocatalysts were prepared by a hydrothermal method. The S-scheme heterojunction can accelerate the recombination of ineffective electron-hole pairs and promote the separation of effective electron-hole pairs, which ultimately improves the photocatalytic activity of the composite catalysts. The composite photocatalyst exhibited excellent photo response in the UV-visible region to achieve highly efficient degradation of organic pollutants, and the degradation rate reached 99.8% after 30 min of light irradiation, which was almost 100% degradation efficiency. In addition, the photocatalyst has excellent magnetic properties and cyclic degradation stability, which helps to separate, recover and recycle the catalyst by magnetic separation technology.
References
- Choi Y, Lee M Y, Kim T H. Evaluating total organic carbon as an indicator for organic pollutant management in the marine environment: A case study on wastewater treatment plant effluent input into the coastal ocean[J]. The Science of the Total Environment, 2024, 919: 170704. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Yu J X, Lu H J, Zhu L Z. Mutation-driven resistance development in wastewater E. coli upon low-level cephalosporins: Pharmacophore contribution and novel mechanism[J]. Water Research, 2024, 252: 121235. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Jentzsch F, Kümmerer K, Olsson O. Status quo on identified transformation products of organic ultraviolet filters and their persistence[J]. International Journal of Cosmetic Science, 2023, 45(Suppl 1): 101-126. [CrossRef] [PubMed] [Google Scholar]
- Gou N, Yang W Y, Gao S, et al. Incorporation of ultrathin porous metal-free graphite carbon nitride nanosheets in polyvinyl chloride for efficient photodegradation[J]. Journal of Hazardous Materials, 2023, 447: 130795. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Qiu H X, Hu J W, Zhang R, et al. The photocatalytic degradation of diesel by solar light-driven floating BiOI/EP composites[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 583: 123996. [CrossRef] [Google Scholar]
- Huang Y J, Yu J X, Wu Z Y, et al. All-inorganic lead halide perovskites for photocatalysis: A review[J]. RSC Advances, 2024, 14(7): 4946-4965. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Di J, Jiang W, Liu Z. Charge-polarized metal site pairs trigger new opportunities in photocatalysis[J]. Materials Today, 2024, 72: 377-391. [CrossRef] [Google Scholar]
- Ni Z N, Wang Q W, Guo Y X, et al. Research progress of tungsten oxide-based catalysts in photocatalytic reactions[J]. Catalysts, 2023, 13(3): 579. [CrossRef] [Google Scholar]
- Zhang H, Wang S Y, Tian J S, et al. Photocatalytic hydrogen peroxide production from seawater over graphitic carbon nitride supported titanium dioxide quantum dots[J]. Journal of Environmental Chemical Engineering, 2024, 12(2): 112290. [CrossRef] [Google Scholar]
- Ullah F, Mohamed N M, Ghani U, et al. First principle DFT + U calculations for the optoelectronic properties of Cu and C-Cu Co-doped TiO2 anatase model[J]. Asian Journal of Chemistry, 2022, 34(7): 1863-1868. [CrossRef] [Google Scholar]
- Kim H I, Kim K, Park S, et al. Titanium dioxide surface modified with both palladium and fluoride as an efficient photocatalyst for the degradation of urea[J]. Separation and Purification Technology, 2019, 209: 580-587. [CrossRef] [Google Scholar]
- Luan N H, Chang C F. Fabrication of Ag3PO4/N-doped TiO2 nanotubes heterojunction photocatalysts for visible-light-driven photocatalysis[J]. Chemosphere, 2024, 350: 141022. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Bezu Z, Taddesse A M, Diaz I. Natural zeolite supported g-C3N4/ZnO/Ag3PO4 composite: A tandem n-n heterojunction for simultaneous photodegradation of dyes under visible and solar irradiation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2024, 449: 115369. [NASA ADS] [CrossRef] [Google Scholar]
- Yu H B, Wang Y L, Wang X H. Synthesis of a Z-scheme photocatalyst (P-doped g-C3N4/Bi3+-doped Ag3PO4) and its photocatalytic performance[J]. Journal of Industrial and Engineering Chemistry, 2024, 130: 436-445. [CrossRef] [Google Scholar]
- Anantharamaiah P N, Manasa K S, Sunil Kumar Y C. Fabrication of magnetically recoverable and reusable MgFe2O4/Ag3PO4 composite for catalytic reduction of 4-nitrophenol[J]. Solid State Sciences, 2020, 106: 106302. [CrossRef] [Google Scholar]
- Zhou T H, Zhang G Z, Yang H, et al. Fabrication of Ag3PO4/GO/NiFe2O4 composites with highly efficient and stable visible-light-driven photocatalytic degradation of rhodamine B[J]. RSC Advances, 2018, 8(49): 28179-28188. [CrossRef] [PubMed] [Google Scholar]
- Hu J, Chen Y, Zhang Y, et al. Fabrication of g-C3N4/Ag/CoFe1.95Eu0.05O4 heterojunctions with enhanced photocatalytic degradation activity[J]. Materials Letters, 2024, 354: 135245. [NASA ADS] [CrossRef] [Google Scholar]
- Chen S K, Jiang D C, Zeng G, et al. Dysprosium doped CoFe2O4 with enhanced magnetic property and photodegradation activity of methyl orange[J]. Materials Letters, 2021, 284: 128966. [NASA ADS] [CrossRef] [Google Scholar]
- Dong L S, Wang Z G, Mi C, et al. Defect-rich hierarchical porous spinel MFe2O4 (M = Ni, Co, Fe, Mn) as high-performance anode for lithium ion batteries[J]. Materials Today Chemistry, 2024, 35: 101853. [CrossRef] [Google Scholar]
- Temerbulatova N T, Tsvetkov M P, Karaivanov D K, et al. Rare earths doped ferrites, characterized by Time Differential γγ Perturbed Angle Correlations method[J]. Journal of Solid State Chemistry, 2019, 277: 281-289. [NASA ADS] [CrossRef] [Google Scholar]
- Zhu H X, Ji Y K, Chen L F, et al. Pt nanowire-anchored dodecahedral Ag3PO4{110} constructed for significant enhancement of photocatalytic activity and anti-photocorrosion properties: Spatial separation of charge carriers and photogenerated electron utilization[J]. Catalysts, 2020, 10(2): 206. [CrossRef] [Google Scholar]
- Jia J K, Huang W X, Feng C S, et al. Fabrication of g-C3N4/Ag3PO4-H2O2 heterojunction system with enhanced visible-light photocatalytic activity and mechanism insight[J]. Journal of Alloys and Compounds, 2019, 790: 616-625. [CrossRef] [Google Scholar]
- Long J L, Xu Y M, Huang W C, et al. Dual-mode optical thermometry based on La2MgTiO6: Mn4+, Dy3+ double perovskite phosphors[J]. Journal of Materials Science: Materials in Electronics, 2023, 34(22): 1613. [NASA ADS] [CrossRef] [Google Scholar]
- Peng H H, Yang J C E, Fu M L, et al. Nanocrystalline ferrihydrite activated peroxymonosulfate for butyl-4-hydroxybenzoate oxidation: Performance and mechanism[J]. Chemosphere, 2020, 242: 125140. [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Kim J, Lim C, Lee D M, et al. Effects of oxygen species in perovskite catalysts on the partial oxidation of methane in a low temperature plasma bed[J]. Journal of Catalysis, 2023, 427: 115116. [CrossRef] [Google Scholar]
- Madona J, Sridevi C, Indumathi N, et al. A novel carbon doped CeO2/g-C3N4 heterostructure for disinfection of microorganisms and degradation of malachite green and amoxicillin under sunlight[J]. Surfaces and Interfaces, 2024, 44: 103803. [CrossRef] [Google Scholar]
- Matussin S N, Khan M M. Phytogenic fabrication of CeO2@SnO2 heterojunction nanostructures for antioxidant studies[J]. Chemical Papers, 2022, 76(4): 2071-2084. [NASA ADS] [CrossRef] [Google Scholar]
- Xu Y, Zhang X W, Zhang Y, et al. Nano flake Ag3PO4 enhanced photocatalytic activity of bisphenol A under visible light irradiation[J]. Colloid and Interface Science Communications, 2020, 37: 100277. [CrossRef] [Google Scholar]
- Zhu P F, Chen Y J, Duan M, et al. Structure and properties of Ag3PO4/diatomite photocatalysts for the degradation of organic dyes under visible light irradiation[J]. Powder Technology, 2018, 336: 230-239. [CrossRef] [Google Scholar]
- Zheng C X, Yang H. Assembly of Ag3PO4 nanoparticles on rose flower-like Bi2WO6 hierarchical architectures for achieving high photocatalytic performance[J]. Journal of Materials Science: Materials in Electronics, 2018, 29(11): 9291-9300. [CrossRef] [Google Scholar]
- Bahrololoomi A, Bilan H K, Podlaha E J. Electrodeposited Ni-Fe onto glassy carbon for the detection of methylene blue[J]. Journal of the Electrochemical Society, 2022, 169(1): 012501. [NASA ADS] [CrossRef] [Google Scholar]
- Yang T, Deng P K, Wang L L, et al. Simultaneous photocatalytic oxygen production and hexavalent chromium reduction in Ag3PO4/C3N4 S-scheme heterojunction[J]. Chinese Journal of Structural Chemistry, 2022, 41(6): 23-38. [Google Scholar]
- Huang Y, Zhang X Y, Zhu G X, et al. Synthesis of silver phosphate/sillenite bismuth ferrite/graphene oxide nanocomposite and its enhanced visible light photocatalytic mechanism[J]. Separation and Purification Technology, 2019, 215: 490-499. [CrossRef] [Google Scholar]
- Khosravani Goshtasb Z, Mehdi Sabzehmeidani M, Ghaedi M, et al. Magnetic Ag3PO4/Ag2CrO4/Fe/Fe3O4 quaternary composite for improved solar-driven photocatalytic degradation of cationic dyes under natural solar radiation[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2022, 428: 113856. [NASA ADS] [CrossRef] [Google Scholar]
- Ma X Q, Li X B, Chen Z, et al. BiOBr/ZnMoO4 step-scheme heterojunction: Construction and photocatalytic degradation properties[J]. Journal of Inorganic Materials, 2023, 38(1): 62. [CrossRef] [Google Scholar]
- Li R, Liu J X, Duo F F, et al. Facile hydrolysis synthesis of novel Bi12O17Br2 photocatalyst with superior reduction ability and photocatalytic activity[J]. Materials Letters, 2018, 224: 5-8. [NASA ADS] [CrossRef] [Google Scholar]
- Song T, Zhang X, Che Q D, et al. Heterojunction nanoarchitectonics with SnS2/g-C3N4 S-scheme toward enhanced photooxidation and photoreduction[J]. Journal of Industrial and Engineering Chemistry, 2022, 113: 389-400. [CrossRef] [Google Scholar]
- Li X B, Luo Q N, Han L, et al. Enhanced photocatalytic degradation and H2 evolution performance of N CDs/S-C3N4 S-scheme heterojunction constructed by π-π conjugate self-assembly[J]. Journal of Materials Science & Technology, 2022, 114: 222-232. [CrossRef] [Google Scholar]
- Feng H G, Zhang C M, Luo M H, et al. A dual S-scheme TiO2@In2Se3@Ag3PO4 heterojunction for efficient photocatalytic CO2 reduction[J]. Nanoscale, 2022, 14(43): 16303-16313. [CrossRef] [PubMed] [Google Scholar]
- Zhu Y K, Zhuang Y, Wang L L, et al. Constructing 0D/1D Ag3PO4/TiO2 S-scheme heterojunction for efficient photodegradation and oxygen evolution[J]. Chinese Journal of Catalysis, 2022, 43(10): 2558-2568. [CrossRef] [Google Scholar]
- Luo C Y, Lin Y, Zhang Y P, et al. S-scheme heterojunction between MOFs and Ag3PO4 leads to efficient photodegradation of antibiotics in swine wastewater[J]. Separation and Purification Technology, 2023, 320: 124052. [CrossRef] [Google Scholar]
- Yao Y, Shen Q H, Chen Q F, et al. Ag/AgX (X = Cl, Br, or I) nanocomposite loaded on Ag3PO4 tetrapods as a photocatalyst for the degradation of contaminants[J]. ACS Applied Nano Materials, 2024, 7(4): 3711-3723. [CrossRef] [Google Scholar]
All Figures
 |
Fig. 1 XRD patterns of different samples (a) and CFDO after magnification (b) |
| In the text | |
 |
Fig. 2 Morphological analysis of the samples (a), (b), (c) SEM images of AP, CFDO and AP/CFDO; (d) HRTEM image of the AP/CFDO; (e) EDS mapping of the AP/CFDO |
| In the text | |
 |
Fig. 3 XPS spectra of the as-prepared AP/CFDO composite sample |
| In the text | |
 |
Fig. 4 Light absorption properties and energy band structure of CFDO, AP and AP/CFDO composites (a) UV-vis diffuse reflectance absorption spectra of the samples; (b) Plots of (αhν)2vs. hν of samples; (c) and (d) VB-XPS curves of AP and CFDO |
| In the text | |
 |
Fig. 5 Fluorescence spectra (a) and EIS profile (b) of samples, and Mott-Schottky curves of AP (c) and CFDO (d) |
| In the text | |
 |
Fig. 6 Visible photocatalytic degradation of MB (a), first-order kinetic curves for different samples (b), and effect of pH (c) and different anions (d) on the degradation efficiency of AP/CFDO |
| In the text | |
 |
Fig. 7 Cyclic degradation stability of the samples (a) Magnetisation curves of the samples (insets are photographs of the solutions before and after magnetic field separation); (b) Cyclic performance curves of the samples for MB degradation; (c) Comparative XRD spectra of AP/CFDO before and after the cyclic degradation experiments |
| In the text | |
 |
Fig. 8 Photocatalytic radical trapping degradation curves (a), ESR spectra of O and ·OH (b), and VB-XPS curves of AP (c) and CFDO (d) and ·OH (b), and VB-XPS curves of AP (c) and CFDO (d)
|
| In the text | |
 |
Fig. 9 Mechanism of AP/CFDO photocatalytic degradation of MB |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


